ENVIRONMENTAL QUALITY AND CONTROL
Achieving and maintaining sterility and overall freedom from contamination of a pharmaceutical product is dependent upon the quality status of the components incorporated,the process utilized,personnel performance,and the environmental conditions under which the process is performed.The standards required for the environmental conditions depend upon the amount of exposure of the CSPto the immediate environment anticipated during processing.The quality and control of environmental conditions for each risk level of operation is explained in this section.In addition,operations using nonsterile components require the use of a method of preparation designed to produce a sterile product.
Critical Site Exposure
The degree of exposure of the product during processing will be affected by the length of time of exposure,the size of the critical site exposed,and the nature of the critical site.
Acritical site is any opening providing a direct pathway between a sterile product and the environment or any surface coming in direct contact with the product and the environment.The risk of such a site picking up contamination from the environment increases with time of exposure.Therefore,the processing plan and the intent of the operator should give due consideration to organization,efficiency,and speed in order to keep such exposure time to a minimum.For example,an ampul should not be opened unnecessarily in advance of use.
The size of the critical site affects the risk of contamination entering the product:the greater the exposed area,the greater the risk.An open vial or bottle exposes to contamination a critical site of much larger area than the tip of a 26-gauge needle.Therefore,the risk of contamination when entering an open vial or bottle is much greater than during the momentary exposure of a needle tip.
The nature of a critical site also affects the risk of contamination.The relatively rough,permeable surface of an elastomeric closure retains microorganisms and other contaminants,after swabbing with an alcohol pad,more readily than does the smooth glass surface of the neck of an ampul.Therefore,the surface disinfection can be expected to be more effective for an ampul.
Once the ampul is open,the critical site of exposure is greatly increased,creating a pathway with the potential for introduction of glass,fiber,and dust into the fluid contained in the ampul.
The prevention or elimination of airborne particles must be given high priority.Airborne contaminants are much more likely to reach critical sites than contaminants that are adhering to the floor or other surfaces below the work level.Further,particles that are relatively large or of high density settle from the airspace more quickly and thus can be removed from the vicinity of critical sites.
Clean Rooms and Barrier Isolators
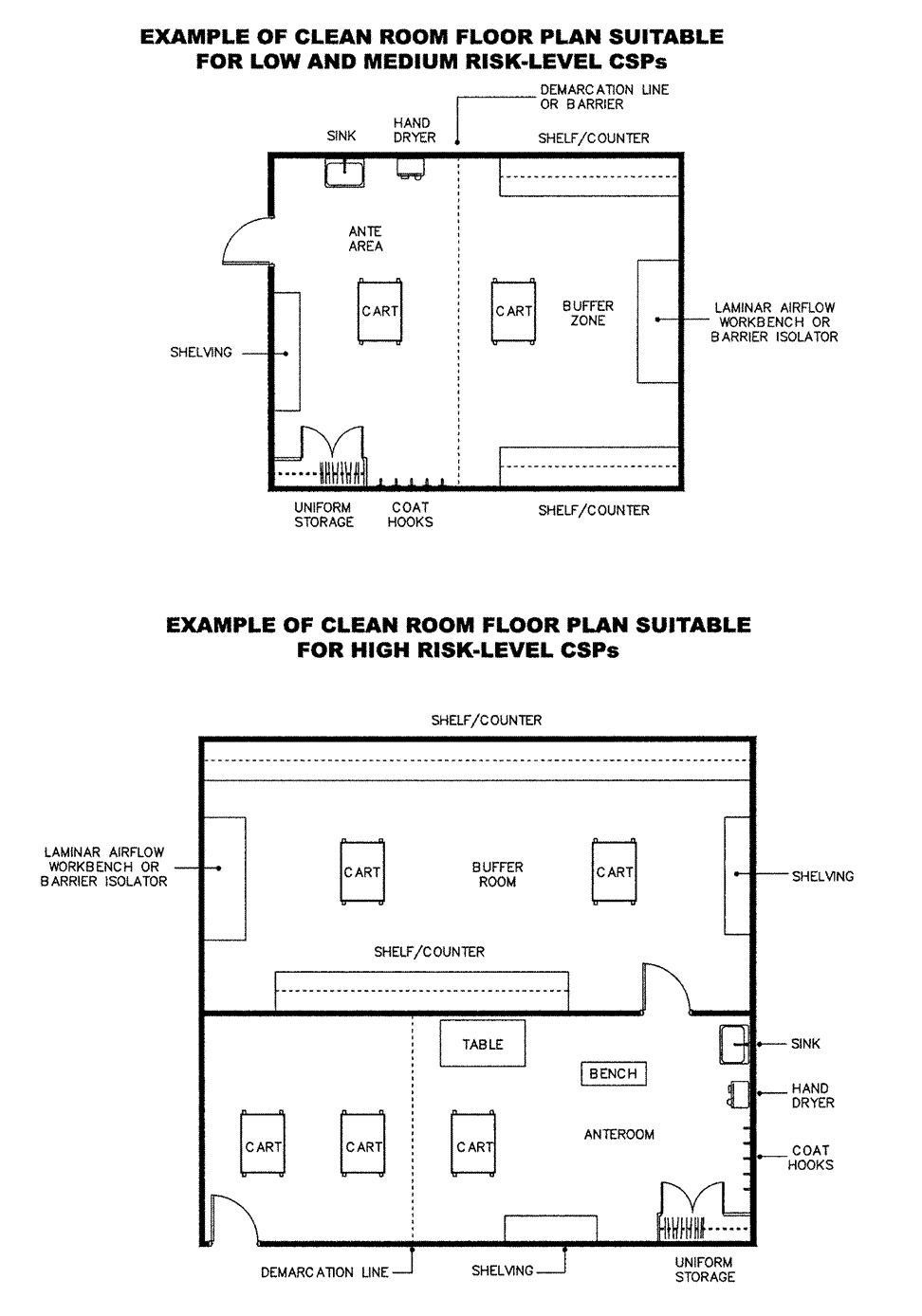
In general,sterile product preparation facilities utilize laminar airflow workbenches (LAFWs)to provide an adequate critical site environment.Adiscussion of the necessary facilities and proper procedures for preparing sterile products using LAFWs in clean rooms is presented below.The use of alternative systems in clean rooms that have been verified to achieve the same or better level of environmental quality as that achieved by properly operated LAFWs may also be utilized.An emerging alternative technology utilizes barrier isolator systems to minimize the extent of personnel contact and interaction,to separate the external environment from the critical site,and to provide an ISO Class 5environment (see Table 1for preparing CSPs.Awell-designed positive pressure barrier isolator,supported by adequate procedures for its maintenance,monitoring,and control,may offer an acceptable alternative to the use of conventional LAFWs in clean rooms for aseptic processing.An example of the arrangement of a clean-room floor plan for low-and medium-risk level CSPs is illustrated in the first drawing in Figure 1.The second drawing in Figure 1depicts an appropriate multicompartment clean-room floor plan for high-risk level CSPs.
Environmental Controls
Engineering controls reduce the potential for airborne contamination in workspaces by limiting the amount and size of contaminants in the CSPprocessing environment.Primary engineering controls are used and generally include horizontal flow clean benches,vertical flow clean benches,biological safety cabinets,and barrier isolators.Primary environmental control must provide at least ISO Class 5quality of air (see Table 1)to which sterile ingredients and components of CSPs are directly exposed.Secondary engineering controls generally provide a buffer zone or buffer room as a core for the location of the workbenches or isolators.
Table 1.International Organization of Standardization (ISO)Classification of Particulate Matter in Room Air [Limits are in particles 0.5µm and larger per cubic meter (current ISO)and cubic feet (former Federal Standard No.209E,FS209E).]*
| Class Name | Particle Size | ||
| ISO Class | U.S.FS209E | ISO,m3 | FS209E,ft.3 |
| 3 | Class 1 | 35.2 | 1 |
| 4 | Class 10 | 352 | 10 |
| 5 | Class 100 | 3520 | 100 |
| 6 | Class 1000 | 35,200 | 1000 |
| 7 | Class 10,000 | 352,000 | 10,000 |
| 8 | Class 100,000 | 3,520,000 | 100,000 |
|
*
Adapted from the Federal Standard No.209E,General Services Administration,Washington,DC,20407(September 11,1992)and ISO[4644-1:1999Clean rooms and associated controlled environments—Part 1:Classification of air cleanliness.For example,3520particles of 0.5µm per m3or larger (ISO Class 5)is equivalent to 100particles per ft3(Class 100)(1m3=34.314ft.3).
|
|||
Airflow through high-efficiency particulate air (HEPA)filters is unidirectional or columnar,and because of the pore size of the filter the “first air”at the face of the filter is,for the purposes of aseptic compounding,free from airborne particulate contamination.Barrier isolators provide a suitable environment by restricting any ambient air from the work chamber.These systems are not as sensitive to external environments as the HEPA-filtered unidirectional airflow units.
Several aspects of barrier isolation and filtered unidirectional airflow in work environment must be understood and practiced in the compounding process.Policies and procedures for maintaining and working in the prescribed conditions for aseptic processing must be prepared,updated,maintained,and implemented and are determined by the scope and risk levels of the activities undertaken in the SPcompounding operation.
In general,the CSPwork environment is designed to have the cleanest work surfaces (horizontal or vertical clean benches,biological safety cabinets,or isolators)located in a buffer area,which is preceded by an anteroom that provides a clean area for donning personnel barriers,such as hair covers,gloves,gowns,or full clean-room attire.The class limit of the buffer or core room has to be demonstrably better than that of ambient air to reduce the risk of contaminants being blown,dragged,or otherwise introduced into the filtered unidirectional airflow environment.For example,strong air currents from opened doors,personnel traffic,or air streams from the heating,ventilating,and air-conditioning systems can easily disrupt the unidirectional,columnar airflow in the open-faced workbenches.The operators may also introduce disruptions in flow by their own movements and by the placement of objects onto the work surface.
Buffer or clean-room areas in which LAFWs are located are to provide at least ISO Class 8air quality (see Table 1).Measuring,weighing,mixing,and other manipulations of nonsterile in-process CSPs are also performed in air quality of at least ISO Class 8(see Table 1).Appropriate air conditioning and humidity controls must be in place for the buffer area.
Tasks carried out within the buffer area should be limited to those for which a controlled environment is necessary.Only the furniture,equipment,supplies,and other goods required for the tasks to be performed may be brought into this room,and they should be nonpermeable,nonshedding,and resistant to disinfectants.Whenever such items are brought into the room,they should first be cleaned and sanitized.Whenever possible,equipment and other items used in the buffer area should not be taken from the room except for calibration,servicing,or other activity associated with the proper maintenance of the item.
The surfaces of ceilings,walls,floors,fixtures,shelving,counters,and cabinets in the buffer area should be smooth,impervious,free from cracks and crevices,and nonshedding,thereby promoting cleanability and minimizing spaces in which microorganisms and other contaminants may accumulate.The surfaces should be resistant to damage by sanitizing agents.Junctures of ceilings to walls should be coved or caulked to avoid cracks and crevices where dirt can accumulate.If ceilings consist of inlaid panels,the panels should be impregnated with a polymer to render them impervious and hydrophobic,and they should be caulked around each perimeter to seal them to the support frame.Walls may be of panels locked together and sealed or of epoxy-coated gypsum board.Preferably,floors are overlaid with wide sheet vinyl flooring with heat-welded seams and coving to the sidewall.Dust-collecting overhangs,such as ceiling utility pipes,or ledges,such as windowsills,should be avoided.The exterior lens surface of ceiling lighting fixtures should be smooth,mounted flush,and sealed.Any other penetrations through the ceiling or walls should be sealed.
The buffer area should contain no sinks or floor drains.Work surfaces should be constructed of smooth,impervious materials,such as stainless steel or molded plastic,so that they are readily cleanable and sanitizable.Carts should be of stainless steel wire or sheet metal construction with good quality,cleanable casters to promote mobility.Storage shelving,counters,and cabinets should be smooth,impervious,free from cracks and crevices,nonshedding,cleanable,and sanitizable.Their number,design,and manner of installation should promote effective cleaning and sanitizing.
CSP Environment
The contamination reduction conditions and procedures in this section include LAFWs being located within buffer or clean-room areas that maintain at least an ISO Class 8(see Table 1).It is preferred,but not necessary,to locate barrier isolators within such a buffer air quality area.The frequency and amount of personnel access to buffer air quality areas is restricted to minimize contaminants,while allowing delivery of essential materials for CSPs.Food,drinks,and materials exposed in patient care and treatment areas must never be introduced into areas where components and ingredients for CSPs are present.
In an area near,but physically isolated from the buffer room area—the anteroom area—supplies,such as needles,syringes,ampuls,bags,vials of parenteral fluids,and packages of transfer tubing sets for large-volume fluids are uncartoned and disinfected.
Hand sanitizing and gowning activities also occur in the anteroom area adjacent to the buffer area.Faucet handles are designed to be hands-free.Before processing CSPs,hands are resanitized after donning all appropriate garb,except for gloves.Ademarcation line or barrier identifies the separation of the buffer area from the anteroom area.Compounding personnel must be capable of accessing the buffer area without use of their hands.Anteroom areas adjacent to buffer areas are intended to minimize the introduction of contaminants into buffer areas.
Cleaning and Sanitizing the Workspaces
The cleaning,sanitizing,and organizing of the direct and contiguous compounding areas (DCCA)is the responsibility of trained operators (pharmacists and technicians)following written procedures and is performed at the beginning of each shift.Before compounding is performed,all items are removed from the DCCAand all surfaces are cleaned of loose material and residue from spills,followed by an application of a residue-free sanitizing agent2that is left on for a time sufficient to exert its antimicrobial effect.
Work surfaces near the DCCAin the buffer or clean area are cleaned in a similar manner,including counter tops and supply carts.Storage shelving is emptied of all supplies and then cleaned and sanitized at least weekly,using approved agents.
Floors in the buffer or clean area are cleaned by mopping once daily when no aseptic operations are in progress.Mopping may be performed by trained and supervised custodial personnel using approved agents described in the written procedures.Only approved cleaning and sanitizing agents are used with careful consideration of compatibilities,effectiveness,and inappropriate or toxic residues.Their schedules of use and methods of application are in accord with written procedures.All cleaning tools,such as wipers,sponges,and mops,are nonshedding and dedicated to use in the buffer or clean area.Floor mops may be used in both the buffer or clean area and anteroom area,but only in that order.Most wipers are discarded after one use.If cleaning tools are reused,their cleanliness is maintained by thorough rinsing and sanitization after use and by storing in a clean environment between uses.Trash is collected in suitable plastic bags and removed with minimal agitation.
In the anteroom area,supplies and equipment removed from shipping cartons are wiped with a sanitizing agent,such as sterile 70%isopropyl alcohol (IPA)3,which is checked periodically for contamination.Alternatively,if supplies are planned to be received in sealed pouches,the pouches can be removed as the supplies are introduced into the buffer or clean area without the need to sanitize the individual supply items.No shipping or other external cartons may be taken into the buffer or clean area.Cleaning and sanitizing of the anteroom area is performed at least weekly by trained and supervised custodial personnel,in accordance with written procedures.However,floors are cleaned and sanitized daily,always proceeding from the buffer or clean area to the anteroom area.Storage shelving is emptied of all supplies and cleaned and sanitized at planned intervals,preferably monthly.
These cleaning and sanitizing procedures apply to both low-risk and high-risk operations.
Personnel Cleansing and Gowning
Personnel are critical keys to the maintenance of asepsis when carrying out their assigned responsibilities.They must be thoroughly trained in aseptic techniques and be highly motivated to maintain these standards each time they prepare a sterile product.
Prior to entering the buffer or clean area,operators should remove outer lab jackets or the like,makeup,and jewelry and should thoroughly scrub hands and arms to the elbow.After drying hands and arms they should properly don clean,nonshedding uniform components,including hair covers,shoe covers,knee-length coats or coveralls,and appropriate protective gloves,in that order.The coats should fit snugly at the wrists and be zipped or snapped closed in the front.Shoe covers should be donned so that feet then touch the floor only on the clean side of the bench or other demarcation.Face masks should be donned just before beginning activities in the DCCAto minimize airborne contaminants from coughing,sneezing,and talking.
When preparing CSPs in a vertical flow LAFWwith a transparent shield between the face of the operator and sterile components,or when using an isolator,wearing a face mask is optional,but head and facial hair must be covered.
Appropriate powder-free protective gloves are sterile or,if nonsterile,are sanitized with an appropriate antimicrobial cleaner such as 70%alcohol before use.Protective gloves are put on as the last uniform component.When nonsterile gloves,chosen for their chemically protective composition,are used,they are disinfected with sterile 70%isopropyl alcohol or an antimicrobial agent that is allowed to evaporate before beginning compounding procedures.Sterile and sanitized gloves do not remain sterile and clean during compounding activities because they come in contact with nonsterile surfaces and air.Therefore,compounding personnel must be trained to avoid touching sterile surfaces of packages,transfer devices,and components within ISO Class 5or superior environments (see Table 1).During protracted compounding activities,personnel should intermittently resanitize their gloves with sterile 70%isopropyl alcohol.
Proper scrubbing and gowning immediately prior to entry into the buffer or clean area is required of all personnel,without exception.Should the operator find it necessary to leave the room,the coat may be carefully removed at the entrance and hung inside out for redonning upon re-entry,but only during the same shift.However,hair covers,masks,shoe covers,and gloves should be discarded and new ones donned prior to re-entry.
For high-risk operations,it is especially critical to minimize the risk of contamination on lab coats,coveralls,and other garb to be worn in the buffer or clean area.Preferably,fresh clean garb should be donned upon each entry into the buffer or clean area to avoid liberating contaminants from previously worn garb.Alternatively,garb that has been worn may be removed with the intention of regarbing for re-entry into the buffer or clean area and stored during the interim under proper control and protection in the anteroom area.Garb worn or taken outside the confines of the anteroom area cannot be worn in the buffer or clean area.
Dispersion of particles from body surfaces,such as from skin rashes,sunburn,or cosmetics,increases the risk of contamination of critical sites and must be appropriately controlled or minimized.If severe,the operator must be excluded from the buffer or clean area until the condition is remedied,especially for high-risk operations.
Suggested Standard Operating Procedures
The pharmacy should have written,properly approved standard operating procedures (SOPs)designed to ensure the quality of the environment in which a CSPis prepared.The following procedures are recommended:
-
Access to the buffer or clean area is restricted to qualified personnel with specific responsibilities or assigned tasks in the area.
-
All cartoned supplies are decontaminated in the anteroom area by removing them from shipping cartons and wiping or spraying with a disinfecting agent,such as sterile IPA,while being transferred to a clean,sanitized cart or other conveyance for introduction into the buffer or clean area.Individual pouched supplies need not be wiped because the pouches can be removed as these supplies are introduced into the buffer or clean area.
-
Supplies required frequently or otherwise needed close at hand but not necessarily needed for the scheduled operations of the shift are decontaminated and stored on the shelving in the anteroom area.
-
Carts used to bring supplies from the storeroom cannot be rolled beyond the demarcation line in the anteroom area,and carts used in the buffer or clean area cannot be rolled outward beyond the demarcation line unless cleaned and sanitized before returning.
-
Generally,supplies required for the scheduled operations of the shift are prepared and brought into the buffer or clean area,preferably on one or more movable carts.Supplies that are required for back-up or general support of operations may be stored on the designated shelving in the buffer or clean area,but avoid excessive accumulation of supplies.
-
Objects that shed particles cannot be brought into the buffer or clean area,including pencils,cardboard cartons,paper towels,and cotton items.Only nonshedding paper-related products (boxes,work records,and so forth)can be brought into the buffer or clean area.
-
Traffic flow in and out of the buffer or clean area must be minimized.
-
Personnel preparing to enter the buffer or clean area must remove all jewelry from hands and arms.
-
Personnel entering the buffer or clean area must first scrub hands and arms with soap,including using a scrub brush on the fingers and nails.An air dryer or disposable nonshedding towels are used to dry hands and arms after washing.
-
Personnel entering the buffer or clean area,after scrubbing,should don attire as described under Personnel Cleansing and Gowning.
-
No chewing gum,candy,or food items may be brought into the buffer or clean area or anteroom area.
-
At the beginning of each compounding activity session,and after liquids are spilled,the surfaces of the direct compounding environment are first cleaned with Purified Waterto remove water soluble residues.Immediately thereafter,the same surfaces are sanitized with sterile 70%isopropyl alcohol,or other effective antimicrobial agents,using a nonlinting wipe.
-
When LAFWs or barrier isolators are used as the ISO Class 5air quality environment (see Table 1),their blowers must be operated continuously during compounding activity,including during interruptions of less than 8hours.When the blower is turned off and before other personnel enter to perform compounding activities,only one person can enter the contiguous buffer area for the purposes of turning on the blower (for at least 30minutes)and of sanitizing the work surfaces.
-
Traffic in the area of the DCCAis minimized and controlled.The DCCAis shielded from all less clean air currents that are of higher velocity than the clean laminar airflow.
-
Supplies to be utilized in the DCCAfor the planned procedures are accumulated and then decontaminated by wiping or spraying the outer surface with IPAor removing the outer wrap at the edge of the DCCAas the item is introduced into the aseptic work area.
-
After proper introduction into the DCCAof supply items required for and limited to the assigned operations,they are so arranged that a clear,uninterrupted path of HEPA-filtered air will bathe all critical sites at all times during the planned procedures.That is,no objects may be placed behind an exposed critical site in a horizontal position or above in the vertical laminar flow workbench.
-
All supply items are arranged in the DCCAso as to reduce clutter and to provide maximum efficiency and order for the flow of work.
-
All procedures are performed in a manner designed to minimize the risk of touch contamination.Gloves are sanitized with adequate frequency with an approved disinfectant.
-
All rubber stoppers of vials and bottles and the neck of ampuls are sanitized with IPAprior to the introduction of a needle or spike for the removal of product.
-
After the preparation of every admixture,the contents of the container are thoroughly mixed and then inspected for the presence of particulate matter,evidence of incompatibility,or other defects.
-
After procedures are completed,used syringes,bottles,vials,and other supplies are removed,but with a minimum of exit and re-entry into the DCCAto minimize the risk of introducing contamination into the aseptic workspace.
Environmental Monitoring
In addition to the evaluation and verification of personnel aseptic techniques and of the adequacy of compounding processes and procedures (see Personnel Training and Evaluation in Aseptic Manipulation Skillssection),assessment and verification of the adequacy of the sterile compounding environment is essential,especially for preparing high-risk preparations.Evaluation of environmental quality is performed by measuring both the total number of particles and the number of viable microorganisms in the controlled air environments of the compounding area.
Certification that each LAFWand barrier isolator is functioning properly and meets the air quality requirement of ISO Class 5(refer to Clean Rooms and Barrier Isolatorsand Table 1in the Environmental Quality and Controlsection)is performed by a qualified operator(s)using current,state-of-the-art electronic air sampling at least every six months and whenever the LAFWor barrier isolator is relocated.Similarly,the air quality of the buffer or clean area and anteroom area is evaluated by a qualified operator(s)for conformance to ISO Class 7and ISO Class 8requirements,as appropriate,at least every six months and when renovations occur.These records are maintained and reviewed by the supervising pharmacist or other designated employee.
Evaluation of airborne microorganisms in the controlled air environments (LAFW,barrier isolators,buffer or clean area,and anteroom area)is performed by properly trained individuals using suitable electric air samplers or by exposing sterile nutrient agar plates for a suitable time frame.For either approach,the air sampling is performed at locations judged by compounding personnel to be the most prone to contamination during compounding activities:this includes zones of air backwash turbulence within LAFWs and other areas where air backwash turbulence may enter the compounding area.Such evaluations are performed as a regular and ongoing process at least monthly for sterile compounding areas used for low-and medium-risk preparations and at least weekly for areas used for high-risk preparations.
For electric air samplers that actively collect volumes of air for evaluation,the instructions for verification and use of these devices must be followed.When using the passive exposure of sterile nutrient agar settling plates,the covers are removed and the media is exposed for a period usually lasting 1hour or longer to collect viable microorganisms as they fall from the environment.At the end of the designated exposure period,the plates are recovered and incubated at a temperature and for a time period conducive to multiplication of microorganisms on the nutrient agar—usually at 30 to 35
to 35 for a minimum of 48hours.The number of discrete colonies of microorganisms are then counted and reported as colony forming units (cfu).This provides a measurement of the level of microbial contamination in the air within the tested environment.
for a minimum of 48hours.The number of discrete colonies of microorganisms are then counted and reported as colony forming units (cfu).This provides a measurement of the level of microbial contamination in the air within the tested environment.
The greatest value of viable microorganism monitored in the air of the compounding environment is realized when normal baseline cfu counts are determined over a period of time.Determining the baseline cfu counts permits identification of a trend toward increasing microbial cfu counts.Asufficiently increasing trend in cfu counts over time must prompt a re-evaluation of the adequacy of cleaning procedures,operational procedures,and air filtration efficiency within the sterile compounding location.Action may be warranted when an increasing trend to 50%above the baseline for areas used for high-and medium-risk preparations or to 100%above baseline for areas used for low-risk preparations is found.
Awritten plan and schedule for the environmental monitoring procedures for airborne microorganisms must be established and followed.The plan must be adequate to evaluate the various controlled air environment areas (LAFW,barrier isolator,buffer or clean area,and anteroom area)of the sterile compounding facility.All compounding personnel are trained in and educated about the importance of this environmental monitoring process.For sterile compounding areas used for low-and medium-risk preparations,a minimum of monthly evaluation is appropriate.For sterile compounding areas used for high-risk preparations,at least weekly evaluation is appropriate.