SYSTEM SUITABILITY
System suitability tests are an integral part of gas and liquid chromatographic methods.They are used to verify that the resolution and reproducibility of the chromatographic system are adequate for the analysis to be done.The tests are based on the concept that the equipment,electronics,analytical operations,and samples to be analyzed constitute an integral system that can be evaluated as such.
The resolution,R,[NOTE—All terms and symbols are defined in the Glossary of Symbols]is a function of column efficiency,N,and is specified to ensure that closely eluting compounds are resolved from each other,to establish the general resolving power of the system,and to ensure that internal standards are resolved from the drug.Column efficiency may be specified also as a system suitability requirement,especially if there is only one peak of interest in the chromatogram;however,it is a less reliable means to ensure resolution than direct measurement.Column efficiency is a measure of peak sharpness,which is important for the detection of trace components.
Replicate injections of a Standard preparation used in the assay or other standard solution are compared to ascertain whether requirements for precision are met.Unless otherwise specified in the individual monograph,data from five replicate injections of the analyte are used to calculate the relative standard deviation,SR,if the requirement is 2.0%or less;data from six replicate injections are used if the relative standard deviation requirement is more than 2.0%.
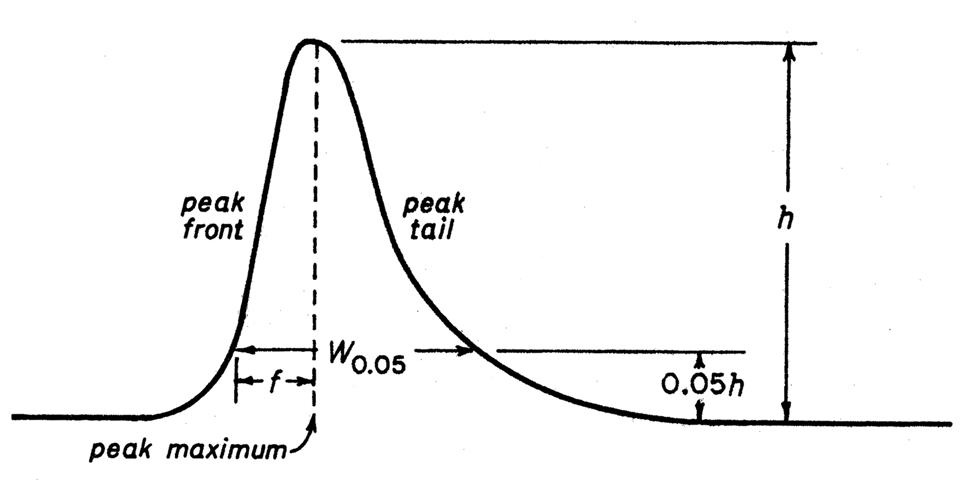
The tailing factor,T,a measure of peak symmetry,is unity for perfectly symmetrical peaks and its value increases as tailing becomes more pronounced (see Fig.2).In some cases,values less than unity may be observed.As peak asymmetry increases,integration,and hence precision,becomes less reliable.
These tests are performed by collecting data from replicate injections of standard or other solutions as specified in the individual monograph.The specification of definitive parameters in a monograph does not preclude the use of other suitable operating conditions (see Procedures under Tests and Assaysin the General Notices).Adjustments of operating conditions to meet system suitability requirements may be necessary.
Unless otherwise directed in the monograph,system suitability parameters are determined from the analyte peak.
To ascertain the effectiveness of the final operating system,it should be subjected to suitability testing.Replicate injections of the standard preparation required to demonstrate adequate system precision may be made before the injection of samples or may be interspersed among sample injections.System suitability must be demonstrated throughout the run by injection of an appropriate control preparation at appropriate intervals.The control preparation can be a standard preparation or a solution containing a known amount of analyte and any additional materials useful in the control of the analytical system,such as excipients or impurities.Whenever there is a significant change in equipment or in a critical reagent,suitability testing should be performed before the injection of samples.No sample analysis is acceptable unless the requirements of system suitability have been met.Sample analyses obtained while the system fails requirements are unacceptable.