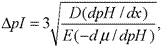ISOELECTRIC FOCUSING
Isoelectric focusing (IEF)is a method of electrophoresis that separates proteins according to their isoelectric points.Separation is carried out in a slab of polyacrylamide or agarose gel that contains a mixture of amphoteric electrolytes (ampholytes).When subjected to an electrical field,the ampholytes migrate in the gel to create a pHgradient.In some cases,gels containing an immobilized pHgradient,prepared by incorporating weak acids and bases to specific regions of the gel network during the preparation of the gel,are used.When the applied proteins reach the gel fraction that has a pHthat is the same as their isoelectric point,their charge is neutralized and migration ceases.Gradients can be made over various ranges of pH,according to the mixture of ampholytes chosen.
General Principles
When a protein is at the position of its isoelectric point,it has no net charge and cannot be moved in a gel matrix by the electric field.It may,however,move from that position by diffusion.The pHgradient forces a protein to remain in its isoelectric point position,thus concentrating it;this concentration effect is called “focusing”.Increasing the applied voltage or reducing the sample load results in improved resolution of bands.The applied voltage is limited by the heat generated because the heat must be dissipated.The use of thin gels and an efficient cooling plate controlled by a thermostatic circulator prevents the burning of the gel while allowing sharp focusing.The separation is estimated by determining the minimum pIdifference,which is necessary to separate two neighboring bands,as follows:
in which Dis the diffusion coefficient of the protein;dpH/dxis the pHgradient;Eis the intensity of the electric field,in volts per centimeter;and –dµ/dpHis the variation of the solute mobility with the pHin the region close to the pI.Since Dand –dµ/dpHfor a given protein cannot be altered,the separation can be improved by using a narrower pHrange and by increasing the intensity of the electric field.
From an operational point,special attention must be paid to sample characteristics and/or preparation.Salt in a sample can be problematic and it is best to prepare the sample,if possible,in deionized water or 2%ampholytes using dialysis or gel filtration if necessary.Potentials of 2500volts have been used and are considered optimal under a given set of conditions.Up to 30watts of constant power can be applied and will generally give complete separation in 1.5to 3.0hours.The time required for completion of focusing in thin-layer polyacrylamide gels is determined by placing a colored protein (e.g.,hemoglobin)at different positions on the gel surface and by applying the electric field:the steady state is reached when all applications give an identical band pattern.In some procedures the completion of the focusing is indicated by the time elapsed after the sample application.
Resolution between protein bands on an IEFgel prepared with carrier ampholytes can be quite good.Better resolution may be achieved by using immobilized pHgradients where the buffering species,which are analogous to carrier ampholytes,are copolymerized within the gel matrix.Proteins exhibiting plsdiffering by as little as 0.02pHunits may be resolved using a gel prepared with carrier ampholytes,while immobilized pHgradients can resolve protein differing by approximately 0.001pHunits.
The IEFgel can be used as an identity test when migration on the gel is compared to a standard preparation and IEFcalibration proteins,the IEFgel can be used as a limit test when the density of a band on IEFis compared subjectively with the density of bands appearing in a standard preparation,or it can be used as a semi-quantitative test when the density is measured using a densitometer or similar instrumentation to determine the relative concentration of protein in the bands.
Apparatus
An apparatus for isoelectric focusing consists of a controllable direct current generator,of stabilized output;a rigid plastic isoelectric focusing chamber that contains a cooled plate of suitable material to support the gel;and a plastic cover with platinum electrodes that are connected to the gel by means of paper wicks of suitable width,length,and thickness,impregnated with solutions of anodic and cathodic electrolytes.
Procedure
Unless otherwise indicated in a given monograph,the following procedure in thick polyacrylamide slab gels is to be used.
Preparation of the Gels—
Assembly—
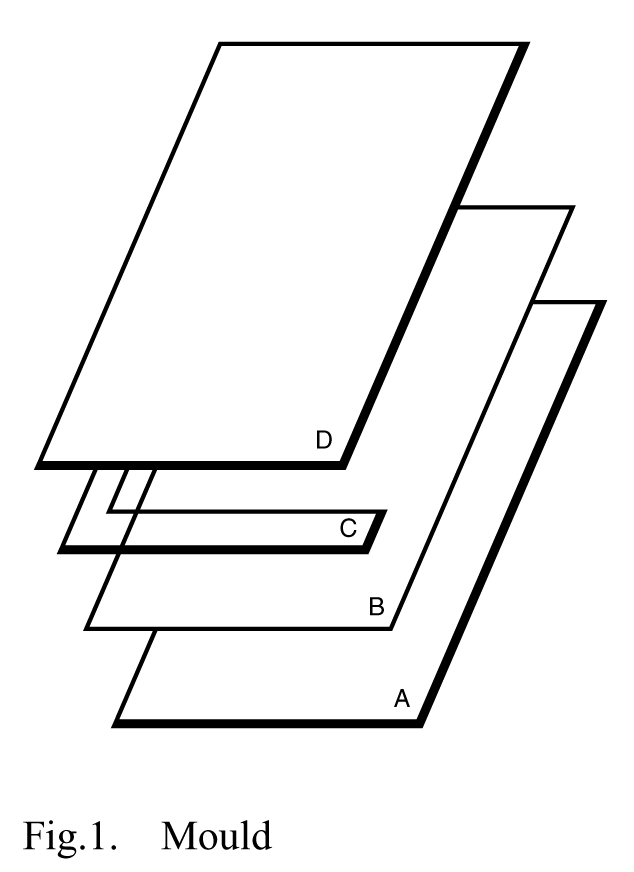
Composed of a glass plate (A)on which a polyester film (B)is placed to facilitate handling of the gel,one or more spacers (C),a second glass plate (D),and clamps to hold the structure together (see Figure 1).
7.5%Polyacrylamide Gel—
Dissolve 29.1g of acrylamide and 0.9g of methylenebisacrylamide in 100mLof water.To 2.5volumes of this solution,add the mixture of ampholytes specified in the individual monograph,and make up to 10volumes with water.Mix carefully,and degas the solution.
Preparation of the Assembly—
Place the polyester film on the lower glass plate,apply the spacer,place the second glass plate,and fit the clamps.Before use,place the mixture on a magnetic stirrer,and add 0.25volumes of a 10%solution of ammonium persulfate and 0.25volumes of tetramethylenediamine.Immediately fill the space between the glass plates of the assembly with the gel.
Fixing Solution for Isoelectric Focusing Polyacrylamide Gel—
Mix 35g of sulfosalicylic acid and 100g of trichloroacetic acid in 1000mLof water.
Coomassie Staining Solution andDestaining Solution—
Use the same solutions indicated in Polyacrylamide Gel Electrophoresis.
Procedure—
Dismantle the assembly,and using the polyester film,transfer the gel onto the cooled support wetted with a few milliliters of a suitable liquid,taking care to avoid forming air bubbles.Prepare the test solutions and reference solutions as specified in the individual monograph.Place strips of paper for sample application,about 10mm ×5mm in size,on the gel,and impregnate each with the prescribed amount of the test and reference solutions.If the protein concentration of the solution is too low,several strips may be superimposed (up to four).Also apply the prescribed quantity of a solution of proteins with known isoelectric points as pHmarkers to calibrate the gel.In some procedures,the gel has pre-cast slots where a solution of the sample is applied instead of using impregnated paper strips.Cut two strips of paper to the length of the gel,and impregnate them with the electrolyte solutions:acid for the anode and alkaline for the cathode.The compositions of the anode and cathode solutions are given in the individual monograph.Apply these paper wicks to each side of the gel several millimeters from the edge.Fit the cover so that the electrodes are in contact with the wicks (with respect to the anodic and cathodic poles).Proceed with the isoelectric focusing by applying the electrical parameters described in the individual monograph.Switch off the current when the migration of the mixture of standard proteins has stabilized.Using forceps,remove the sample application strips and the two electrode wicks.Immerse the gel in Fixing Solution for Isoelectric Focusing Polyacrylamide Gel.Incubate with gentle shaking at room temperature for 30minutes.Drain off the solution,and add 200mLof Destaining Solution.Incubate with shaking for 1hour.Drain the gel,add Coomassie Staining Solution.Incubate for 30minutes.Destain the gel by passive diffusion with Destaining Solutionuntil the bands are well visualized against a clear background.Locate the position and intensity of the bands in the electropherogram as prescribed in the individual monograph.
Alternative Procedure—
When a monograph references the general method for isoelectric focusing above,variations in methodology or procedure may be used,subject to validation.These variations include the use of commercially available pre-cast gels;the use of immobilized pHgradients;the use of rod gels;and the use of cassettes of different dimensions,including ultra-thin (0.2mm)gels;the variations in the sample application procedure,including different sample volumes or the use of sample application masks or wicks other than paper;the use of alternate running conditions,including variations in the electric field depending on gel dimensions and equipment,and the use of fixed migration times rather than subjective interpretation of band stability;the inclusion of a pre-focusing step;the use of automated instrumentation;and the use of agarose gels.
Validation of Procedure
Where alternative methods to the general method are employed,they must be validated.The following criteria may be used to validate the separation:the formation of a stable pHgradient of desired characteristics,evaluated using colored pHmarkers of known isoelectric points;the comparison with electropherogram provided with the chemical reference substance for the preparation to be examined;and any other validation criteria as prescribed in the individual monograph.
Specified Variations to the General Method
Variations to the general method required for the analysis of specific substances may be specified in detail in individual monographs.Variations may include the addition of urea in the running gel (3Mconcentration is often satisfactory to keep protein in solution but up to 8Mcan be used).Some proteins precipitate at their isoelectric point.In this case,urea is included in the gel formulation to keep the protein in solution.If urea is used,only fresh solutions should be used to prevent carbamylation of the protein.Other variations include the use of alternative staining methods and the use of gel additives such as non-ionic detergents (e.g.,octylglucoside)or zwitterionic detergents (e.g.,CHAPSor CHAPSO)to prevent proteins from aggregating or precipitating.
NOTE—The following are general precautionary items that can be used to improve the method.Samples can be applied to any area on the gel,but in general,they should be applied to areas where they are expected to focus.To protect the proteins from extreme pHenvironments,samples should not be applied close to either electrode.During method development,the analyst can try applying the protein in three positions on the gel (i.e.,middle and both ends);the pattern of a protein applied at opposite ends of the gel may not be identical.Aphenomenon known as cathodic drift,where the pHgradient decays over time,may occur if a gel is focused too long.Although not well understood,electroendoosmosis and absorption of carbon dioxide may be factors that lead to cathodic drift.Cathodic drift is observed as focused protein migrating off the cathode end of the gel.Immobilized pHgradients may be used to address this problem.Efficient cooling (approximately 4 )of the bed that the gel lies on during focusing is important.High field strengths used during isoelectric focusing can lead to overheating and affect the quality of the focused gel.
)of the bed that the gel lies on during focusing is important.High field strengths used during isoelectric focusing can lead to overheating and affect the quality of the focused gel.