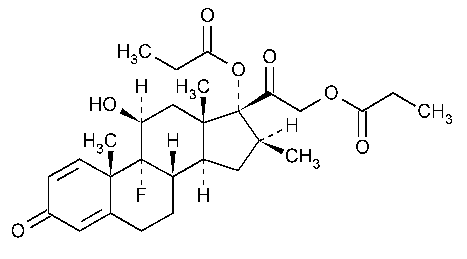
Betamethasone Dipropionate
Pregna-1,4-diene-3,20-dione,9-fluoro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-,(11b,16b).
9-Fluoro-11b,17,21-trihydroxy-16b-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate [5593-20-4].
»Betamethasone Dipropionate contains not less than 97.0percent and not more than 103.0percent of C28H37FO7,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.Store at 25 ,excursions permitted between 15
,excursions permitted between 15 and 30
and 30 .
.
USP Reference standards á11ñ—
USP Beclomethasone Dipropionate RS.USP Betamethasone Dipropionate RS.USP Betamethasone Valerate RS.
Identification—
A:Infrared Absorption á197Mñ.
Test solution:
1mg per mL,in chloroform.
Developing solvent system:
a mixture of chloroform and acetone (7:1).
Specific rotation á781Sñ:
between +63 and +70
and +70 .
.
Test solution:
10mg per mL,in dioxane.
Loss on drying á731ñ:
Dry it at 105 for 3hours:it loses not more than 1.0%of its weight.
for 3hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.2%,a platinum crucible being used.
Chromatographic purity—
Mobile phase—
Prepare a filtered and degassed mixture of acetonitrile and water (65:35).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
System suitability solution—
Dissolve accurately weighed quantities of USP Betamethasone Dipropionate RSand USP Betamethasone Valerate RSin Mobile phaseto obtain a solution having final concentrations of about 0.05mg of each per mL.
Test solution—
Transfer about 3mg of Betamethasone Dipropionate,accurately weighed,to a suitable flask.Add 10mLof Mobile phase,and shake until dissolved.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm ×15-cm column that contains packing L1.The flow rate is about 1mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the resolution,R,between betamethasone valerate and betamethasone dipropionate is not less than 4.0;and the column efficiency is not less than 8000theoretical plates.
Procedure—
Inject a volume (about 10µL)of the Test solutioninto the chromatograph,record the chromatogram,and measure all the peak responses.Calculate the percentage of each impurity in the portion of Betamethasone Dipropionate taken by the formula:
100(ri/rs),
in which riis the peak response for each impurity;and rsis the sum of the responses for all the peaks:not more than 1.0%of any individual impurity is found;and not more than 2.0%of total impurities is found.
Assay—
Mobile phase—
Prepare a suitable acetonitrile solution (about 1in 2),degassed by ultrasonic vibration for 5to 10minutes,such that the retention time of betamethasone dipropionate is approximately 14minutes and that of beclomethasone dipropionate is approximately 18minutes.[NOTE—Do not leave the mobile phase in the column overnight,but flush the system after use with water for 15minutes,followed by methanol for 15minutes.]
Internal standard solution—
Prepare a solution of USP Beclomethasone Dipropionate RSin a solution of acetic acid in methanol (1in 1000)having a known concentration of about 0.9mg per mL.
Standard preparation—
Prepare a solution of USP Betamethasone Dipropionate RSin a solution of acetic acid in methanol (1in 1000)having a known concentration of about 0.6mg per mL.Transfer 5.0mLof this solution to a suitable vial,and add 5.0mLof Internal standard solutionto obtain a solution having known concentrations of about 0.3mg of betamethasone dipropionate and about 0.45mg of beclomethasone dipropionate per mL.
Assay preparation—
Accurately weigh about 60mg of Betamethasone Dipropionate.Dilute quantitatively and stepwise with a solution of acetic acid in methanol (1in 1000)to obtain a solution containing about 0.6mg per mL.Transfer 5.0mLof this solution to a suitable vial,and add 5.0mLof Internal standard solution.
Procedure—
Separately inject equal volumes (between 5µLand 25µL)of the Assay preparationand the Standard preparationinto a high-pressure liquid chromatograph (see Chromatography á621ñ)operated at room temperature,by means of a suitable microsyringe or sampling valve,adjusting the specimen size and other operating parameters such that the peak obtained from the internal standard in the Standard preparationis about 0.6full-scale.Typically,the apparatus is fitted with a 4-mm ×30-cm column that contains packing L1,and is equipped with a UVdetector capable of monitoring absorption at 254nm or 240nm and a suitable recorder,and is capable of operating at a column pressure of up to 3500psi.In a suitable chromatogram,the lowest and highest peak area ratios (RS)of three successive injections of the Standard preparationagree within 2.0%.Determine the ratio of the peak heights,at equivalent retention times,obtained with the Assay preparationand the Standard preparation,and calculate the quantity,in mg,of C28H37FO7in the portion of Betamethasone Dipropionate taken by the formula:
200C(RU/RS),
in which Cis the concentration,in mg per mL,of USP Betamethasone Dipropionate RSin the Standard preparation;and RUand RSare the peak height ratios of betamethasone dipropionate to the internal standard obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 246
Pharmacopeial Forum:Volume No.29(5)Page 1428
Phone Number:1-301-816-8139