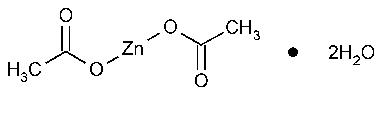
Zinc Acetate
Acetic acid,zinc salt,dihydrate.
Zinc acetate dihydrate [5970-45-6].
Anhydrous 183.48 [557-34-6].
»Zinc Acetate contains not less than 98.0percent and not more than 102.0percent of C4H6O4Zn·2H2O.
Packaging and storage—
Preserve in tight containers.
Identification—
Asolution (1in 20)responds to the tests for Zinc á191ñand for Acetate á191ñ.
pHá791ñ:
between 6.0and 8.0,in a solution (1in 20).
Insoluble matter—
A20-g portion,dissolved in 150mLof water containing 1mLof glacial acetic acid,shows not more than 1.0mg of insoluble matter (0.005%).
Arsenic,Method Iá211ñ:
3ppm.
Lead á251ñ—
Dissolve 0.5g in 1mLof a mixture of equal parts,by volume,of nitric acid and water in a separator.Add 3mLof Ammonium Citrate Solutionand 0.5mLof Hydroxylamine Hydrochloride Solution,and render alkaline,with ammonium hydroxide,to phenol red TS.Add 10mLof Potassium Cyanide Solution,and immediately extract the solution with successive 5-mLportions of Dithizone Extraction Solution,draining off each extract into another separator,until the last portion of dithizone solution retains its green color.Shake the combined extracts for 30seconds with 20mLof dilute nitric acid (1in 100),and discard the chloroform layer.Add to the acid solution 4.0mLof Ammonia-Cyanide Solutionand 2drops of Hydroxylamine Hydrochloride Solution.Add 10.0mLof Standard Dithizone Solution,and shake the mixture for 30seconds.Filter the chloroform layer through acid-washed filter paper into a color-comparison tube,and compare the color with that of a standard prepared as follows:to 20mLof dilute nitric acid (1in 100)add 0.01mg of lead,4mLof Ammonia-Cyanide Solution,and 2drops of Hydroxylamine Hydrochloride Solution,and shake for 30seconds with 10.0mLof Standard Dithizone Solution.Filter through acid-washed filter paper into a color-comparison tube:the color of the sample solution does not exceed that of the control (0.002%).
Chloride á221ñ—
A1.5-g portion shows no more chloride than corresponds to 0.10mLof 0.020Nhydrochloric acid (0.005%).
Sulfate á221ñ—
A1.0-g portion shows no more sulfate than corresponds to 0.10mLof 0.020Nsulfuric acid (0.010%).
Alkalies and alkaline earths—
Dissolve 2.0g in about 150mLof water contained in a 200-mLvolumetric flask,add sufficient ammonium sulfide TSto precipitate the zinc completely,dilute with water to volume,and mix.Filter through a dry filter,rejecting the first portion of the filtrate.To 100mLof the subsequent filtrate add 5drops of sulfuric acid,evaporate to dryness,and ignite:the weight of the residue does not exceed 2mg (0.2%).
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Dissolve about 400mg of Zinc Acetate,accurately weighed,in 100mLof water.Add 5mLof ammonia–ammonium chloride buffer TSand 0.1mLof eriochrome black TS,and titrate with 0.05Medetate disodium VSuntil the solution is deep blue in color.Each mLof 0.05Medetate disodium is equivalent to 10.98mg of C4H6O4Zn·2H2O.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 2052
Phone Number:1-301-816-8305