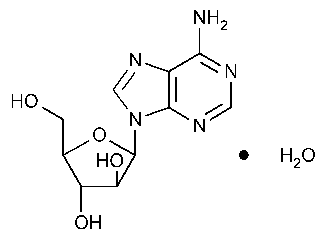
Vidarabine
9H-Purin-6-amine,9-b-D-arabinofuranosyl-,monohydrate.
9-b-D-Arabinofuranosyladenine monohydrate [24356-66-9].
Anhydrous 267.25 [5536-17-4].
»Vidarabine has a potency equivalent to not less than 845µg and not more than 985µg of C10H13N5O4per mg.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable or other sterile dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable or other sterile forms.
Identification,Infrared Absorption á197Kñ.
Specific rotation á781Sñ:
between -56.0 and -65.0
and -65.0 (l=365nm).
(l=365nm).
Test solution:
10mg of anhydrous vidarabine per mL,in dimethylformamide.
Bacterial endotoxins á85ñ—
Where the label states that Vidarabine is sterile or must be subjected to further processing during the processing of injectable dosage forms,it contains not more than 0.5USP Endotoxin Unit per mg of vidarabine.Where it is intended for use in preparing ophthalmic dosage forms,it is exempt from the requirements.
Sterility á71ñ—
Where the label states that Vidarabine is sterile,it meets the requirements when tested as directed for Direct Inoculation of the Culture Mediumunder Test for Sterility of the Product to be Examined,except to transfer 2g of solid specimen to each test medium.
Loss on drying á731ñ—
Dry about 100mg in vacuum at 100 and at a pressure not exceeding 5mm of mercury for 4hours:it loses between 5.0%and 7.0%of its weight.
and at a pressure not exceeding 5mm of mercury for 4hours:it loses between 5.0%and 7.0%of its weight.
Assay—
Mobile phase—
Dissolve 2.2g of docusate sodium in 10mLof glacial acetic acid and 500mLof methanol in a 1000-mLvolumetric flask.Dilute with water to volume,and mix.Pass this solution through a membrane filter having a 1-µm or finer porosity.
Standard preparation—
Dissolve about 24mg ofUSP Vidarabine RS,accurately weighed,in 150mLof water in a 200-mLvolumetric flask by heating to 100 for 10minutes.Cool,dilute with water to volume,and mix.
for 10minutes.Cool,dilute with water to volume,and mix.
Assay preparation—
Using Vidarabine,prepare as directed forStandard preparation.
Chromatographic system
(see Chromatography á621ñ)—The chromatograph is equipped with a 254-nm detector and a 4-mm ×30-cm column that contains packing L1.Chromatograph three replicate injections of the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation is not more than 3.0%.
Procedure—
Introduce equal volumes (approximately 10µL)of the Assay preparationand theStandard preparationinto the instrument,operated at room temperature,by means of a suitable microsyringe or sampling valve.Adjust the operating conditions so that satisfactory chromatography and peak responses are obtained.Use a detector sensitivity setting that gives a peak height for vidarabine that is at least 50%of scale.Measure peak responses at the same retention times obtained with theAssay preparationand the Standard preparation.Calculate the potency,in µg of C10H13N5O4per mg,of the Vidarabine taken by the formula:
F(rU/rS)(WS/WU),
in which Fis the potency ofUSP Vidarabine RS,in µg of vidarabine per mg;rUand rSare the peak responses obtained from theAssay preparationand theStandard preparation,respectively;and WUandWSare the amounts,in mg,of USP Vidarabine RSand Vidarabine taken,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 2023
Phone Number:1-301-816-8335