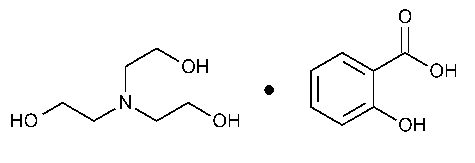
Trolamine Salicylate
»Trolamine Salicylate is a compounded mixture of Trolamine and Salicylic Acid in propylene glycol.It contains not less than 95.0percent and not more than 105.0percent of the labeled amount of C13H21NO6.
Packaging and storage—
Preserve in tight containers in a cool place.
Identification,Ultraviolet Absorption á197Uñ—
Solution:
1mg per mL,in 0.1-cm cells.
Medium:
methanol.
Absorptivities do not differ by more than 1.0%.
Specific gravity á841ñ:
between 1.190and 1.220.
Refractive index á831ñ:
between 1.505and 1.535at 20 .
.
pHá791ñ:
between 6.5and 7.5,in a 50mg per mLsolution in water.
Limit of free salicylic acid—
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture.
Test solution—
Transfer about 48mg of Trolamine Salicylate,accurately weighed,to a 50-mLvolumetric flask,dilute with xylene to volume,and mix.
Standard solution 1—
Dissolve an accurately weighed quantity of USP Salicylic Acid RSin xylene,and dilute quantitatively,and stepwise if necessary,with xylene to obtain a solution having a known concentration of about 0.2mg per mL.
Standard solution 2—
Dilute an accurately measured quantity of Standard solution 1in xylene to obtain a solution having a known concentration of about 0.1mg per mL.
Application volume:
5µLof each solution.
Developing solvent system:
a mixture of toluene,acetone,and glacial acetic acid (17:8:0.2).
Procedure—
Proceed as directed for Thin-Layer Chromatographyunder Chromatography á621ñ.Develop in a chamber previously equilibrated with Developing solvent system.Examine the plate under long-wavelength UVlight.Any secondary spot obtained from the Test solutionis not greater in size or intensity than the spot obtained from Standard solution 1:not more than 0.02%of free salicylic acid is found.
Chromatographic purity—
Mobile phase,Standard preparation,andChromatographic system—
Proceed as directed in the Assay.
Test preparation—
Use the Assay preparation.
Procedure—
Inject a volume (about 10µL)of the Test preparationinto the chromatograph,record the chromatogram,and measure all of the peak responses.Calculate the percentage of each impurity in the portion of Trolamine Salicylate taken by the formula:
100(ri/rs),
in which riis the peak response for each impurity,and rsis the sum of the responses of all the peaks:not more than 1.0%of any individual impurity is found,and not more than 2.0%of total impurities is found.
Assay—
Mobile phase—
Prepare a filtered and degassed mixture of water and acetonitrile (7:3).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity of USP Salicylic Acid RSin methanol,and dilute quantitatively,and stepwise if necessary,with methanol to obtain a solution having a known concentration of about 48µg per mL.
Assay preparation—
Transfer a portion of Trolamine Salicylate,equivalent to about 300mg of C13H21NO6,accurately weighed,to a 250-mLvolumetric flask,and dilute with methanol to volume.Transfer 2mLof this solution to a 50-mLvolumetric flask,dilute with methanol to volume,and mix.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 308-nm detector and a 4.0-mm ×12.5-cm column that contains packing L1.The flow rate is about 1mLper minute.The column temperature is maintained at 30 .Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the column efficiency is not less than 8000theoretical plates;the tailing factor is not more than 1.5;and the relative standard deviation for replicate injections is not more than 2.0%.
.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the column efficiency is not less than 8000theoretical plates;the tailing factor is not more than 1.5;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the percentage of C13H21NO6in the portion of Trolamine Salicylate taken by the formula:
100(rU/rS),
in which rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Daniel K.Bempong,Ph.D.,Scientist
Expert Committee:(PA2)Pharmaceutical Analysis 2
USP28–NF23Page 1996
Pharmacopeial Forum:Volume No.30(4)Page 1312
Phone Number:1-301-816-8143