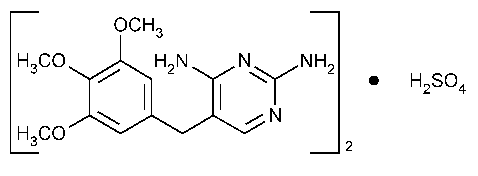
Trimethoprim Sulfate
2,4-Pyrimidinediamine,5-[(3,4,5-trimethoxyphenyl)methyl]-,sulfate (2:1)(salt).
2,4-Diamino-5-[(3,4,5-trimethoxybenzyl)pyrimidine]-,sulfate (2:1)(salt) [56585-33-2].
»Trimethoprim Sulfate contains not less than 98.5percent and not more than 101.0percent of (C14H18N4O3)2·H2SO4,calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed containers.Store at 25 ,excursions permitted between 15
,excursions permitted between 15 and 30
and 30 .
.
USP Reference standards á11ñ—
USP Trimethoprim RS.USP3-Anilino-2-(3,4,5-trimethoxybenzyl)acrylonitrile RS.
Identification—
A:Ultraviolet Absorption á197Uñ—
Solution—
Transfer about 100mg of it,accurately weighed,to a 100-mLvolumetric flask,dissolve in 25mLof alcohol,dilute with 0.1Nsodium hydroxide to volume and mix.
Medium—
Dilute the Solutionquantitatively and stepwise with 0.1Nsodium hydroxide to obtain a solution containing a known concentration of about 20µg per mL.
Absorptivity,at about 287nm,calculated on the anhydrous basis,is between 83.0%and 86.4%of the USP Trimethoprim RS.
B:
It responds to the tests for Sulfate á191ñ.
pHá791ñ:
between 7.5and 8.5,in a solution (0.5mg per mL).
Water,Method Iá921ñ:
not more than 3.0%.
Chromatographic purity—
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture.
Diluent—
Prepare a mixture of chloroform and methanol (9:1).
Test solution—
Transfer about 20mg of Trimethoprim Sulfate,accurately weighed,to a 10-mLvolumetric flask,add 4mLof glacial acetic acid,and swirl to dissolve.Dilute with Diluentto volume,and mix.
Standard solution—
Dissolve an accurately weighed quantity of USP Trimethoprim RSin Diluent.Dilute an accurately measured volume of this solution quantitatively,and stepwise if necessary,with Diluentto obtain a solution having a known concentration of 0.02mg per mL.
Application volume:
10µL.
Developing solvent system:
a mixture of chloroform,methanol,and 6Nammonium hydroxide (95:7.5:1).
Procedure—
Proceed as directed for Thin-Layer Chromatographyunder Chromatography á621ñ.Spray the plate with a freshly prepared mixture of 1.9g of ferric chloride in 20mLof water and 0.5g of potassium ferricyanide in 10mLof water.Compare the intensities of any secondary spots observed in the chromatogram of the Test solutionwith that of the principal spot in the chromatogram of the Standard solution:no secondary spot in the chromatogram obtained from the Test solutionis larger or more intense than the principal spot obtained from the Standard solution(0.1%);and the sum of the intensities of the secondary spots obtained from the Test solutioncorresponds to not more than 0.5%.
Assay—
Transfer about 800mg of Trimethoprim Sulfate,accurately weighed,to a 50-mLconical flask,add about 60mLof glacial acetic acid,and titrate with 0.1Nperchloric acid VS,determining the endpoint potentiometrically.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 67.87mg of (C14H18N4O3)2·H2SO4.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1988
Pharmacopeial Forum:Volume No.29(6)Page 1995
Phone Number:1-301-816-8394