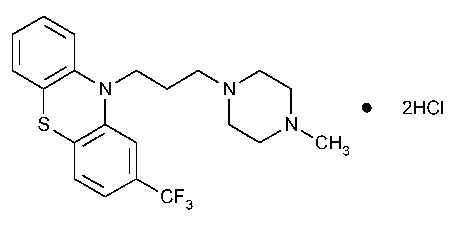
Trifluoperazine Hydrochloride
10H-Phenothiazine,10-[3-(4-methyl-1-piperazinyl)propyl-[2-(trifluoromethyl)-,dihydrochloride.
10-[3-(4-Methyl-1-piperazinyl)propyl-[2-(trifluoromethyl)phenothiazine dihydrochloride [440-17-5].
»Trifluoperazine Hydrochloride,dried in vacuum at 60 for 4hours,contains not less than 98.0percent and not more than 101.0percent of C21H24F3N3S·2HCl.
for 4hours,contains not less than 98.0percent and not more than 101.0percent of C21H24F3N3S·2HCl.
Change to read:
Packaging and storage—
Preserve in tight,light-resistant containers.
USP Reference standards á11ñ—
USP Trifluoperazine Hydrochloride RS.
NOTE—Throughout the following procedures,protect test or assay specimens,the Reference Standard,and solutions containing them,by conducting the procedures without delay,under subdued light,or by using low-actinic glassware.
Identification—
A:
Infrared Absorption á197Mñ.
B:
Ultraviolet Absorption á197Uñ—
Solution:
10µg per mL.
Medium:
0.1Nhydrochloric acid.
Absorptivities at 255nm,calculated on the dried basis,do not differ by more than 2.0%.
C:
Asolution (1in 100)responds to the tests for Chloride á191ñ.
D:
Prepare a solution in methanol containing 1.2mg per mL.Apply 5µLeach of this solution and a solution of USP Trifluoperazine Hydrochloride RSin methanol containing 1.2mg per mLto a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel.Allow the spots to dry,and develop the chromatogram in a solvent system consisting of a mixture of acetone and ammonium hydroxide (200:1)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and allow the solvent to evaporate.Locate the spots on the plate by lightly spraying with a solution of iodoplatinic acid prepared by dissolving 100mg of chloroplatinic acid in 1mLof 1Nhydrochloric acid,adding 25mLof potassium iodide solution (1in 25),diluting with water to 100mL,and then adding 0.5mLof formic acid:the RFvalue of the principal spot from the test solution corresponds to that from the Standard solution.
pHá791ñ:
between 1.7and 2.6,in a solution (1in 20).
Loss on drying á731ñ—
Dry it in vacuum at 60 for 4hours:it loses not more than 1.5%of its weight.
for 4hours:it loses not more than 1.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Dissolve about 500mg of Trifluoperazine Hydrochloride,previously dried and accurately weighed,in 50mLof glacial acetic acid,and add crystal violet TSand 15mLof mercuric acetate TS.Titrate with 0.1Nperchloric acid VSto a blue-green endpoint.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 24.02mg of C21H24F3N3S·2HCl.
Auxiliary Information—
Staff Liaison:Ravi Ravichandran,Ph.D.,Senior Scientist
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 1974
Pharmacopeial Forum:Volume No.29(6)Page 1993
Phone Number:1-301-816-8330