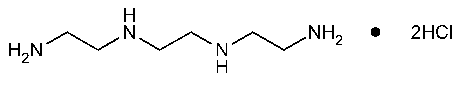
Trientine Hydrochloride
1,2-Ethanediamine,N,N¢-bis(2-aminoethyl)-,dihydrochloride.
Triethylenetetramine dihydrochloride [38260-01-4].
»Trientine Hydrochloride contains not less than 97.0percent and not more than 103.0percent of C6H18N4·2HCl,calculated on the dried basis.
Packaging and storage—
Preserve under an inert gas in tight,light-resistant containers,and store in a refrigerator.
Identification,Infrared Absorption á197Mñ.
pHá791ñ:
between 7.0and 8.5,in a solution (1in 100).
Loss on drying á731ñ—
Dry it in vacuum at a pressure not exceeding 5mm of mercury at 40 for 4hours:it loses not more than 2.0%of its weight.
for 4hours:it loses not more than 2.0%of its weight.
Residue on ignition á281ñ:
not more than 0.15%.
Heavy metals,Method IIá231ñ:
0.001%.
Chromatographic purity—
The sum of the intensities of all secondary spots obtained from the Test preparationin Part Iand Part IIcorresponds to not more than 2.0%.
Part I—
Spray reagent—
Dissolve 300mg of ninhydrin in a mixture of 100mLof butyl alcohol and 3mLof glacial acetic acid.
Standard preparation A—
[NOTE—Use low-actinic glassware.]Dissolve an accurately weighed quantity of USP Trientine Hydrochloride RSin methanol to obtain a solution containing 10mg per mL.
Standard preparation B—
[NOTE—Use low-actinic glassware.]Dissolve an accurately weighed quantity of diethylenetriamine in methanol to obtain a solution containing 1.0mg per mL.Transfer 3.0mLof this solution to a 100-mLvolumetric flask,dilute with methanol to volume,and mix.
Standard preparation C—
[NOTE—Use low-actinic glassware.]Dissolve an accurately weighed quantity of 1-(2-aminoethyl)piperazine in methanol to obtain a solution containing 1.0mg per mL.Transfer 10.0mLof this solution to a 100-mLvolumetric flask,dilute with methanol to volume,and mix.
Standard preparation D—
[NOTE—Use low-actinic glassware.]Transfer 5.0mLof Standard preparation Cto a 10-mLvolumetric flask,dilute with methanol to volume,and mix.
Test preparation—
[NOTE—Use low-actinic glassware.]Dissolve an accurately weighed quantity of Trientine Hydrochloride in methanol to obtain a solution containing 10mg per mL.
Procedure—
Apply separately 3µLeach of the Test preparation,of Standard preparation B,and of Standard preparation Cto a suitable unwashed,high performance thin-layer chromatographic plate (see Chromatography á621ñ)having a 1.5-cm preadsorbent zone and coated with a 0.15-mm layer of chromatographic silica gel mixture.To a fourth spot,apply 3µLeach of Standard preparations A,B,and C.To a fifth spot,apply 3µLeach of Standard preparations A,B,and D.Allow the spots to dry,place the plate in a chromatographic chamber,and develop the chromatograms in a solvent system consisting of a mixture of isopropyl alcohol and ammonium hydroxide (3:2)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and dry the plate with the aid of a current of air.Spray the plate with Spray reagent,dry at 105 for 5minutes,and observe the plate under long-wavelength UVlight.Determine the locus of the diethylenetriamine and the 1-(2-aminoethyl)piperazine spots from the chromatograms of Standard preparations Band C,respectively.Determine the concentration of diethylenetriamine in the Test preparationby comparing the size and intensity of any secondary spot from the chromatogram of the Test preparationhaving an RFvalue corresponding to the RFvalue of diethylenetriamine with the diethylenetriamine spots obtained from the chromatograms of the Standard preparationmixtures.Determine the concentration of any other observed impurities in the Test preparationby comparing the size and intensity of any other secondary spots from the chromatogram of the Test preparationwith the 1-(2-aminoethyl)piperazine spots obtained from the chromatograms of the Standard preparationmixtures.
for 5minutes,and observe the plate under long-wavelength UVlight.Determine the locus of the diethylenetriamine and the 1-(2-aminoethyl)piperazine spots from the chromatograms of Standard preparations Band C,respectively.Determine the concentration of diethylenetriamine in the Test preparationby comparing the size and intensity of any secondary spot from the chromatogram of the Test preparationhaving an RFvalue corresponding to the RFvalue of diethylenetriamine with the diethylenetriamine spots obtained from the chromatograms of the Standard preparationmixtures.Determine the concentration of any other observed impurities in the Test preparationby comparing the size and intensity of any other secondary spots from the chromatogram of the Test preparationwith the 1-(2-aminoethyl)piperazine spots obtained from the chromatograms of the Standard preparationmixtures.
Part II—
Spray reagent—
Dissolve 200mg of ninhydrin in 100mLof alcohol.
Tris
(2-aminoethyl)amine stock solution—[NOTE—Use low-actinic glassware.]Dissolve an accurately weighed quantity of tris(2-aminoethyl)amine in methanol to obtain a solution containing 1.0mg per mL.
Standard preparation A—
[NOTE—Use low-actinic glassware.]Dissolve an accurately weighed quantity of USP Trientine Hydrochloride RSin methanol to obtain a solution containing 10mg per mL.
Standard preparation B—
[NOTE—Use low-actinic glassware.]Transfer 1.0mLof Tris(2-aminoethyl)amine stock solutionto a 10-mLvolumetric flask,dilute with methanol to volume,and mix.
Standard preparation C—
[NOTE—Use low-actinic glassware.]Transfer 0.5mLof Tris(2-aminoethyl)amine stock solutionto a 10-mLvolumetric flask,dilute with methanol to volume,and mix.
Test preparation—
[NOTE—Use low-actinic glassware.]Dissolve an accurately weighed quantity of Trientine Hydrochloride in methanol to obtain a solution containing 10mg per mL.
Procedure—
Apply separately 3µLeach of the Test preparationand of Standard preparation Ato a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture and previously washed with methanol.To a third spot apply 3µLeach of Standard preparations Aand B.To a fourth spot,apply 3µLeach of Standard preparations Aand C.Allow the spots to dry,place the plate in a chromatographic chamber,and develop the chromatograms in a solvent system consisting of a mixture of ammonium hydroxide and alcohol (2:1)at a temperature of 2 to 6
to 6 until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and dry the plate with the aid of a current of air.Spray the plate with Spray reagent,dry at 105
until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and dry the plate with the aid of a current of air.Spray the plate with Spray reagent,dry at 105 for 5minutes,and observe the plate under long-wavelength UVlight.Determine the concentration of tris(2-aminoethyl)amine in the Test preparationby comparing the size and intensity of any secondary spot from the chromatogram of the Test preparationhaving an RFvalue corresponding to the RFvalue of tris(2-aminoethyl)amine with the tris(2-aminoethyl)amine spots obtained from the chromatograms of the Standard preparationmixtures.
for 5minutes,and observe the plate under long-wavelength UVlight.Determine the concentration of tris(2-aminoethyl)amine in the Test preparationby comparing the size and intensity of any secondary spot from the chromatogram of the Test preparationhaving an RFvalue corresponding to the RFvalue of tris(2-aminoethyl)amine with the tris(2-aminoethyl)amine spots obtained from the chromatograms of the Standard preparationmixtures.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Dissolve about 220mg of Trientine Hydrochloride,accurately weighed,in 150mLof water in a 250-mLbeaker.Adjust with hydrochloric acid to a pHof 2.0;then adjust with ammonium hydroxide to a pHof 9.5±0.5;and then adjust with glacial acetic acid to a pHof 5.0.Heat the solution to 90 ,and while hot,titrate with 0.1Ncupric nitrate VS,determining the endpoint potentiometrically,using an electrode system consisting of a cupric ion-selective electrode and a calomel reference electrode with an outer filling solution of 1Mpotassium nitrate.Perform a blank determination (see Titrimetry á541ñ),and make any necessary correction.Each mLof 0.1Ncupric nitrate is equivalent to 21.92mg of C6H18N4·2HCl.
,and while hot,titrate with 0.1Ncupric nitrate VS,determining the endpoint potentiometrically,using an electrode system consisting of a cupric ion-selective electrode and a calomel reference electrode with an outer filling solution of 1Mpotassium nitrate.Perform a blank determination (see Titrimetry á541ñ),and make any necessary correction.Each mLof 0.1Ncupric nitrate is equivalent to 21.92mg of C6H18N4·2HCl.
Auxiliary Information—
Staff Liaison:Elena Gonikberg,Ph.D.,Scientist
Expert Committee:(PA4)Pharmaceutical Analysis 4
USP28–NF23Page 1973
Phone Number:1-301-816-8251