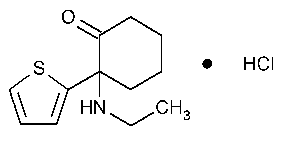
Tiletamine Hydrochloride
Cyclohexanone,2-(ethylamino)-2-(2-thienyl)-.
2-(Ethylamino)-2-(2-thienyl)cyclohexanone hydrochloride [14176-50-2].
»Tiletamine Hydrochloride contains not less than 97.0percent and not more than 103.0percent of C12H17NOS·HCl.
Packaging and storage—
Preserve in tight containers.
Labeling—
Label it to indicate that it is for veterinary use only.Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Clarity of solution—
Dissolve 1.0g of it in 10mLof water:the solution is clear.
Identification—
A:
Infrared Absorption á197Kñ.
B:
Ultraviolet Absorption á197Uñ—
Solution:
0.3mg per mL.
Medium:
0.1Nhydrochloric acid.
Absorptivities at 234nm do not differ by more than 3.0%.
C:
It meets the requirements of the tests for Chloride á191ñ.
Bacterial endotoxins á85ñ—
Where the label states that Tiletamine Hydrochloride is sterile or must be subjected to further processing during the preparation of injectable dosage forms,it contains not more than 0.07USP Endotoxin Unit per mg of tiletamine.
Sterility á71ñ—
Where the label states that Tiletamine Hydrochloride is sterile,it meets the requirements when tested as directed for Membrane Filtrationunder Test for Sterility of the Product to be Examined.
pHá791ñ:
between 3.0and 5.0,in a solution (1in 10).
Water,Method Iá921ñ:
not more than 1.0%.
Residue on ignition á281ñ:
not more than 0.5%.
Heavy metals,Method IIá231ñ:
0.002%.
Chromatographic purity—
Prepare a test solution of Tiletamine Hydrochloride in methanol containing 50.0mg per mL.Prepare a Standard solution in methanol containing 1.0mg of USP Tiletamine Hydrochloride RSper mL.Prepare a thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture as follows.Prewash the plate by developing it in a mixture of methanol and ether (8:2)to the top of the plate,and allow the plate to dry.Separately apply 5µLof the test solution and the Standard solution to the plate,and develop the chromatogram until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the chamber,mark the solvent front,allow the plate to air-dry,and examine under short-and long-wavelength UVlight:no individual secondary spot observed in the chromatogram obtained from the test solution is greater in size or intensity than the principal spot observed in the chromatogram obtained from the Standard solution,corresponding to 2%,and the total of any such spots observed does not exceed 3%.
Chloride content—
Transfer about 250mg of it,accurately weighed,to a conical flask,add 5mLof water,5mLof glacial acetic acid,and 50mLof methanol,and swirl to dissolve.Add 1drop of eosin Y TS,and titrate with 0.1Nsilver nitrate VSto the endpoint when the granular precipitate first turns to a permanent pink color.Each mLof 0.1Nsilver nitrate is equivalent to 3.545mg of chloride:between 13.24%and 14.06%is found.
Assay—
Transfer about 300mg of Tiletamine Hydrochloride,accurately weighed,to a conical flask,add 70mLof glacial acetic acid and 10mLof mercuric acetate TS,and swirl to dissolve.Add 2drops of crystal violet TS,and titrate with 0.1Nperchloric acid VSto a blue-green endpoint.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 25.98mg of C12H17NOS·HCl.
Auxiliary Information—
Staff Liaison:Ian DeVeau,Ph.D.,Senior Scientist
Expert Committee:(VET)Veterinary Drugs
USP28–NF23Page 1930
Phone Number:1-301-816-8178