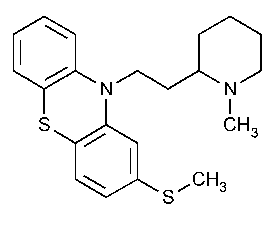
Thioridazine
10H-Phenothiazine,10-[2-(1-methyl-2-piperidinyl)ethyl]-2-(methylthio)-.
10-[2-(1-Methyl-2-piperidyl)ethyl]-2-(methylthio)phenothiazine [50-52-2].
»Thioridazine contains not less than 99.0percent and not more than 101.0percent of C21H26N2S2,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed,light-resistant containers.
USP Reference standards á11ñ—
USP Thioridazine RS.
NOTE—Throughout the following procedures,protect test,or assay specimens,the Reference Standard,and solutions containing them,by conducting the procedures without delay,under subdued light,or using low-actinic glassware.
Identification,Infrared Absorption á197Kñ.
Loss on drying á731ñ—
Dry it in vacuum at 50 for 4hours:it loses not more than 0.5%of its weight.
for 4hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Chromatographic purity—
[NOTE—Conduct this procedure without delay,under subdued light.]Transfer 100mg of Thioridazine to a 10-mLvolumetric flask,add a mixture of methanol and ammonium hydroxide (49:1)to volume,and mix to obtain the Test solution.Using an accurately weighed quantity of USP Thioridazine RS,prepare two solutions in the same solvent system containing 50µg per mL(Solution A,equivalent to 0.5%)and 20µg per mL(Solution B,equivalent to 0.2%).Apply 5-µLportions of the Test solutionand each of the two Standard solutions to a thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Immediately develop the chromatogram in a solvent system consisting of a mixture of chloroform,isopropyl alcohol,and ammonium hydroxide (74:25:1)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,allow the solvent to evaporate,and examine the plate under short-wavelength UVlight:the chromatograms show principal spots at about the same RFvalue;no secondary spot,if present in the chromatogram from the Test solution,is more intense than the principal spot obtained from Solution A(0.5%);and the sum of the intensities of all secondary spots,if present in the chromatogram from the Test solution,is not greater than 0.5%.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay—
Dissolve about 300mg of Thioridazine,accurately weighed,in 60mLof glacial acetic acid,and titrate with 0.1Nperchloric acid VS,determining the endpoint potentiometrically.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 37.06mg of C21H26N2S2.
Auxiliary Information—
Staff Liaison:Ravi Ravichandran,Ph.D.,Senior Scientist
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 1916
Phone Number:1-301-816-8330