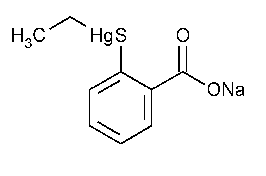
Thimerosal
Mercury,ethyl(2-mercaptobenzoato-S)-,sodium salt.
Ethyl (sodium o-mercaptobenzoato)mercury [54-64-8].
»Thimerosal contains not less than 97.0percent and not more than 101.0percent of C9H9HgNaO2S,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:
Infrared Absorption á197Kñ.
B:
To a solution (1in 100)add a few drops of silver nitrate TS:a white precipitate is formed.
Loss on drying á731ñ—
Dry it in vacuum over phosphorus pentoxide to constant weight:it loses not more than 0.5%of its weight.
Ether-soluble substances—
Shake 500mg,accurately weighed,with 20mLof anhydrous ethyl ether for 10minutes.Filter,evaporate the ether in a tared container,dry the residue in vacuum over phosphorus pentoxide,and weigh:the weight of residue does not exceed 4mg (0.8%).
Mercury ions—
Iodide reagent—
[NOTE—Prepare fresh daily;keep the stopper in the flask and protect from light.]Dissolve 33.20g of potassium iodide in 75mLof water in a 100-mLvolumetric flask,dilute with water to volume,and mix.
Standard preparation—
Transfer about 190mg of mercuric chloride,accurately weighed,to a 200-mLvolumetric flask,and dissolve in 100mLof water.Dilute with water to volume,and mix.Transfer 5.0mLof this solution to a 50-mLvolumetric flask,dilute with water to volume,and mix.The concentration of mercuric chloride in the Standard preparationis about 95µg per mL.
Test solution—
Transfer about 500mg of Thimerosal,accurately weighed,to a 100-mLvolumetric flask,add water to volume,and mix to dissolve.
Test preparation A—
Transfer 10.0mLof the Test solutionto a 50-mLvolumetric flask,dilute with water to volume,and mix.
Test preparation B—
Transfer 10.0mLof the Test solutionto a 50-mLvolumetric flask,add 5.0mLof the Standard preparation,dilute with water to volume,and mix.
Procedure—
[NOTE—Protect all solutions from light prior to determining their absorbances.]Label five 10-mLvolumetric flasks C,D,E,F,and R.Transfer 5.0mLof Test preparation Ato flasks Cand D,transfer 5.0mLof Test preparation Bto flasks Eand F,and transfer 5.0mLof water to flask R.Dilute flasks Cand Ewith water to volume,and mix.Dilute flasks D,F,and Rwith the Iodide reagentto volume,and mix.Concomitantly determine the absorbances of the solutions in 1-cm cells at the wavelength of maximum absorbance for the tetraiodomercurate ion at about 323nm (located from the similarly determined UVspectrum of a solution prepared by mixing 1.0mLof Standard preparationwith 5.0mLof Iodide reagentand diluting with water to 10.0mL),with a suitable spectrophotometer,using water as the blank.Record the absorbances of the solutions in flasks C,D,E,F,and Ras AC,AD,AE,AF,and AR,respectively.Calculate the percentage of mercury ions by the formula:
(200.59/271.50)(5C/W)(AU/AS),
in which 200.59is the atomic weight of mercury,271.50is the molecular weight of mercuric chloride,AUis the absorbance of the test specimen obtained by the equation:
AU=(AD-AR-AC),
ASis the absorbance of the Standard obtained by the equation:
AS=(AF-AR-AE-AU),
Cis the concentration,in µg per mL,of mercuric chloride in the Standard preparation,and Wis the weight,in mg,of Thimerosal taken:the limit is 0.70%.
Readily carbonizable substances á271ñ—
Dissolve about 200mg in 5mLof sulfuric acid TS:the solution has no more color than Matching Fluid J.
Assay—
[NOTE—The Standard preparation and the Assay preparation may be diluted quantitatively with water,if necessary,to obtain solutions of suitable concentrations adaptable to the linear or working range of the instrument.]
Standard preparation—
Dissolve an accurately weighed quantity of USP Thimerosal RSin water,and dilute quantitatively with water to obtain a solution having a known concentration of about 1mg per mL.
Assay preparation—
Transfer about 100mg of Thimerosal,accurately weighed,to a 100-mLvolumetric flask,add 25mLof water,and mix to dissolve.Dilute with water to volume,and mix.
Procedure—
Concomitantly determine the absorbances of the Standard preparationand the Assay preparation,at the mercury resonance line of 254nm,with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering á851ñ),equipped with a mercury hollow-cathode lamp and an air–acetylene flame,using water as the blank.Calculate the quantity,in mg,of C9H9HgNaO2Sin the portion of Thimerosal taken by the formula:
100C(AU/AS),
in which Cis the concentration,in mg per mL,of USP Thimerosal RSin the Standard preparation;and AUand ASare the absorbances of the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1911
Phone Number:1-301-816-8394