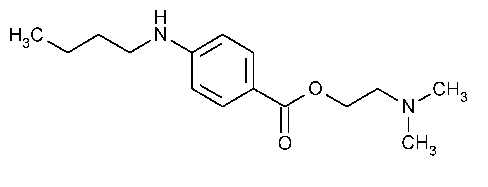
Tetracaine
Benzoic acid,4-(butylamino)-,2-(dimethylamino)ethyl ester.
2-(Dimethylamino)ethyl p-(butylamino)benzoate [94-24-6].
»Tetracaine contains not less than 98.0percent and not more than 101.0percent of C15H24N2O2,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:
Dissolve 100mg in 10mLof dilute hydrochloric acid (1in 120),and add 1mLof potassium thiocyanate solution (1in 4):a crystalline precipitate is formed.Recrystallize the precipitate from water,and dry at 80 for 2hours:it melts between 130
for 2hours:it melts between 130 and 132
and 132 (see Melting Range or Temperature á741ñ).
(see Melting Range or Temperature á741ñ).
B:
Dissolve about 90mg,accurately weighed,in 10mLof dilute hydrochloric acid (1in 120)in a 500-mLvolumetric flask,dilute with water to volume,and mix.Transfer 5.0mLof this solution to a 100-mLvolumetric flask,add 2mLof Buffer No.6,10percent,pH6.0(see Phosphate Buffers á81ñ),dilute with water to volume,and mix:the UVabsorption spectrum of the solution so obtained exhibits maxima and minima at the same wavelengths as that of a 1in 100,000solution of USP Tetracaine Hydrochloride RSin a mixture of water and Buffer No.6(50:1),10percent,pH6.0(see Phosphate Buffers á81ñ),and the respective molar absorptivities,calculated on the dried basis,at the wavelength of maximum absorbance at about 310nm do not differ by more than 2.0%.[NOTE—The molecular weight of tetracaine hydrochloride (C15H24N2O2·HCl)is 300.82.]
Loss on drying á731ñ—
Dry it in vacuum over phosphorus pentoxide for 18hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Chromatographic purity—
Dissolve an accurately weighed quantity of Tetracaine in chloroform to obtain a test solution containing 50mg per mL.Prepare a Standard solution of 4-(butylamino)benzoic acid in methanol containing 0.2mg per mL.Apply separate 5-µLportions of the test solution and the Standard solution to a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Develop the plate in a suitable chromatographic chamber containing a solvent system consisting of a mixture of chloroform,methanol,and isopropylamine (98:7:2)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the chamber,and dry in a current of warm air.Examine the plate under short-wavelength UVlight:any spot obtained from the test solution,other than the principal spot,is not more intense than the principal spot obtained from the Standard solution (0.4%),and the sum of the intensities of any such spots is not greater than 0.8%.
Assay—
Transfer about 500mg of Tetracaine,accurately weighed,to a suitable vessel.Add 5mLof hydrochloric acid and 50mLof water,cool to 15 ,add about 25g of crushed ice,and slowly titrate with 0.1Msodium nitrite VS,stirring vigorously,until a glass rod dipped into the titrated solution produces an immediate blue ring when touched to starch iodide paper.When the titration is complete,the endpoint is reproducible after the mixture has been allowed to stand for 1minute.Perform a blank determination,and make any necessary correction.Each mLof 0.1Msodium nitrite is equivalent to 26.44mg of C15H24N2O2.
,add about 25g of crushed ice,and slowly titrate with 0.1Msodium nitrite VS,stirring vigorously,until a glass rod dipped into the titrated solution produces an immediate blue ring when touched to starch iodide paper.When the titration is complete,the endpoint is reproducible after the mixture has been allowed to stand for 1minute.Perform a blank determination,and make any necessary correction.Each mLof 0.1Msodium nitrite is equivalent to 26.44mg of C15H24N2O2.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 1879
Phone Number:1-301-816-8379