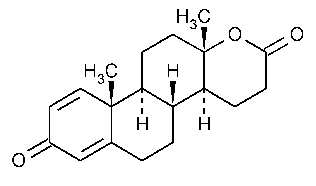
Testolactone
D-Homo-17a-oxaandrosta-1,4-diene-3,17-dione.
13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid d-lactone [968-93-4].
»Testolactone contains not less than 95.0percent and not more than 105.0percent of C19H24O3,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
Infrared Absorption á197Kñ.
Solution:
10µg per mL.
Medium:
methanol.
Specific rotation á781Sñ:
between -44 and -52
and -52 .
.
Test solution:
12.5mg per mL,in chloroform.
Loss on drying á731ñ—
Dry it in vacuum at 100 for 3hours:it loses not more than 1.0%of its weight.
for 3hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.003%.
Chromatographic purity—
Standard preparation—
Transfer 10mg of USP Testolactone RS,accurately weighed,to a 50-mLvolumetric flask,dissolve in acetone,dilute with acetone to volume,and mix.
Test preparation—
Transfer 250mg of Testolactone,accurately weighed,to a 50-mLvolumetric flask,dissolve in acetone,dilute with acetone to volume,and mix.
Procedure—
Coat a 20-×20-cm thin-layer chromatographic plate (see Chromatography á621ñ)with a 0.25-mm layer of chromatographic silica gel mixture,dry for 15minutes at room temperature,heat at 105 for 1hour,and cool in a desiccator.Divide the area of the plate into three approximately equal sections,the left and right sections to be used for the Test preparationand the Standard preparation,respectively,and the center section for the blank.Apply 10µLof the Standard preparationand 20µLof the Test preparation2.5cm from the bottom of the designated sections of the plate,and dry the spots with a current of air.Develop the chromatogram in a solvent system consisting of a mixture of butyl acetate and acetone (4:1)until the solvent front has moved to within about 1cm of the top of the plate.Remove the plate from the developing chamber,mark the solvent front,and allow the solvent to evaporate.Locate the spots on the plate by viewing under short-wavelength UVlight:the RFvalue of the principal spot obtained from the Test preparationcorresponds to that obtained from the Standard preparation,not more than two impurities are found in the chromatogram of the Test preparation,and the size and color of the spot representing any impurity obtained from the Test preparationare not greater or more intense than those of the principal spot obtained from the Standard preparation.
for 1hour,and cool in a desiccator.Divide the area of the plate into three approximately equal sections,the left and right sections to be used for the Test preparationand the Standard preparation,respectively,and the center section for the blank.Apply 10µLof the Standard preparationand 20µLof the Test preparation2.5cm from the bottom of the designated sections of the plate,and dry the spots with a current of air.Develop the chromatogram in a solvent system consisting of a mixture of butyl acetate and acetone (4:1)until the solvent front has moved to within about 1cm of the top of the plate.Remove the plate from the developing chamber,mark the solvent front,and allow the solvent to evaporate.Locate the spots on the plate by viewing under short-wavelength UVlight:the RFvalue of the principal spot obtained from the Test preparationcorresponds to that obtained from the Standard preparation,not more than two impurities are found in the chromatogram of the Test preparation,and the size and color of the spot representing any impurity obtained from the Test preparationare not greater or more intense than those of the principal spot obtained from the Standard preparation.
Ordinary impurities á466ñ—
Test solution:
methanol.
Standard solution:
methanol.
Eluant:
a mixture of butyl acetate and acetone (4:1).
Visualization:
6.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay—
Isoniazid reagent—
Dissolve 1.0g of isoniazid in about 500mLof methanol,add 1.25mLof hydrochloric acid,dilute with methanol to 1000mL,and mix.
Standard preparation—
Dissolve a suitable quantity of USP Testolactone RS,accurately weighed,in chloroform,and prepare,by quantitative,and stepwise dilution if necessary,a solution in chloroform having a known concentration of about 30µg per mL.
Assay preparation—
Transfer about 60mg of Testolactone,accurately weighed,to a 100-mLvolumetric flask,dissolve in chloroform,dilute with chloroform to volume,and mix.Transfer 5.0mLof this solution to a second 100-mLvolumetric flask,dilute with chloroform to volume,and mix.
Procedure—
Transfer 5.0mLeach of the Standard preparation,the Assay preparation,and chloroform to provide the blank,to separate 25-mLvolumetric flasks,add 10.0mLof Isoniazid reagentto each flask,and mix.Place the flasks in a water bath maintained at a temperature of 55±2 ,and allow them to stand for 70minutes.Cool,dilute each solution with chloroform to volume,and mix.Determine the absorbances of the solutions in 1-cm cells at the wavelength of maximum absorbance at about 415nm,with a suitable spectrophotometer,against the blank.Calculate the quantity,in mg,of C19H24O3in the Testolactone taken by the formula:
,and allow them to stand for 70minutes.Cool,dilute each solution with chloroform to volume,and mix.Determine the absorbances of the solutions in 1-cm cells at the wavelength of maximum absorbance at about 415nm,with a suitable spectrophotometer,against the blank.Calculate the quantity,in mg,of C19H24O3in the Testolactone taken by the formula:
2C(AU/AS),
in which Cis the concentration,in µg per mL,of USP Testolactone RSin the Standard preparation,and AUand ASare the absorbances of the solutions from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(PA6)Pharmaceutical Analysis 6
USP28–NF23Page 1874
Phone Number:1-301-816-8389