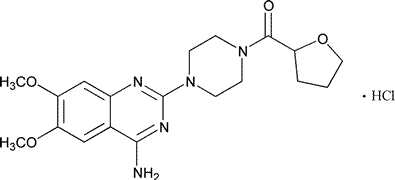
Terazosin Hydrochloride
C19H25N5O4·HCl·2H2O
459.92
Piperazine,1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-[(tetrahydro-2-furanyl)carbonyl]-,monohydrochloride,dihydrate.
1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(tetrahydro-2-furoyl)piperazine monohydrochloride dihydrate [70024-40-7].
Piperazine,1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-[(tetrahydro-2-furanyl)carbonyl]-,monohydrochloride,dihydrate.
1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(tetrahydro-2-furoyl)piperazine monohydrochloride dihydrate [70024-40-7].
Anhydrous 423.89 [63074-08-8].
»Terazosin Hydrochloride contains not less than 98.0percent and not more than 102.0percent of C19H25N5O4·HCl,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers,and store at a temperature between 20 and 25
and 25 .
.
USP Reference standards á11ñ—
USP Terazosin Hydrochloride RS.USP Terazosin Related Compound A RS.USP Terazosin Related Compound B RS.USP Terazosin Related Compound C RS.
Color and clarity of solution—
Dissolve a quantity of Terazosin Hydrochloride in methanol solution (90in 100)to obtain a 1in 100solution:this solution is clear and colorless to pale yellow,when compared to methanol solution (90in 100).
Identification—
B:
The retention time of the major peak in the chromatogram of theAssay preparation corresponds to that in the chromatogram of theStandard preparation,as obtained in theAssay.
C:
It meets the requirements of the tests forChloride á191ñ,a solution prepared by dissolving 100mg in 10mLof methanol solution (90in 100)being examined.
Loss on drying á731ñ—
Dry it in vacuum at 105 for 3hours:it loses not more than 9.0%of its weight.
for 3hours:it loses not more than 9.0%of its weight.
Residue on ignition á281ñ:
not more than 0.2%,determined on a 1.0-g specimen.
Heavy metals,Method IIá231ñ:
0.002%.
Limit of tetrahydro-2-furancarboxylic acid—
Blank solution—
Transfer 2.0mLof glacial acetic acid to a 100-mLvolumetric flask,dilute with acetone to volume,and mix.Mix 5.0mLof this solution and 5.0mLof acetone;pass through a nylon membrane filter having a 0.45-µm or finer porosity,previously washed with acetone;and discard the first 1mLof the filtrate.
Internal standard solution—
Transfer about 100mg of capric acid,accurately weighed,to a 100-mLvolumetric flask;dissolve in and dilute with acetone to volume;and mix.Transfer 10.0mLof this solution and 2.0mLof glacial acetic acid to a 100-mLvolumetric flask,dilute with acetone to volume,and mix.
Standard stock solution—
Dissolve an accurately weighed amount of tetrahydro-2-furancarboxylic acid in acetone to obtain a solution having a known concentration of about 1.0mg per mL.Dilute with acetone quantitatively,and stepwise if necessary,to obtain a solution having a known concentration of about 100µg per mL.
Standard solution—
Transfer 5.0mLof theStandard stock solution and 5.0mLofInternal standard solution to a 50-mLcentrifuge tube,and mix.Pass through a nylon membrane filter having a 0.45-µm or finer porosity,previously washed with acetone;and discard the first 1mLof the filtrate.
Test solution—
Transfer about 100mg of Terazosin Hydrochloride,accurately weighed,to a 50-mLcentrifuge tube;add 5.0mLof acetone and 5.0mLofInternal standard solution;and shake for about 30minutes.Centrifuge for about 10minutes;pass through a nylon membrane filter having a 0.45-µm or finer porosity,previously washed with acetone;and discard the first 1mLof the filtrate.
Chromatographic system(see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm ×10-m fused-silica capillary column coated with a 1.2-µm film of liquid phase G25.The column temperature is maintained at about 170 .The injection port is configured for splitless injection,and its temperature is maintained at about 230
.The injection port is configured for splitless injection,and its temperature is maintained at about 230 .The detector temperature is maintained at about 240
.The detector temperature is maintained at about 240 .The carrier gas is helium,flowing at a rate of about 9mLper minute.Chromatograph theBlank solution,and measure the peak responses as directed forProcedure:ensure that there are no extraneous peaks.Chromatograph theStandard solution,and measure the peak responses as directed forProcedure:the relative retention times are 1.0for tetrahydro-2-furancarboxylic acid and 1.2for capric acid;the resolution,R,between tetrahydro-2-furancarboxylic acid and capric acid is not less than 2.3;and the relative standard deviation,determined from the peak response ratios of tetrahydro-2-furancarboxylic acid to capric acid for replicate injections is not more than 6.5%.
.The carrier gas is helium,flowing at a rate of about 9mLper minute.Chromatograph theBlank solution,and measure the peak responses as directed forProcedure:ensure that there are no extraneous peaks.Chromatograph theStandard solution,and measure the peak responses as directed forProcedure:the relative retention times are 1.0for tetrahydro-2-furancarboxylic acid and 1.2for capric acid;the resolution,R,between tetrahydro-2-furancarboxylic acid and capric acid is not less than 2.3;and the relative standard deviation,determined from the peak response ratios of tetrahydro-2-furancarboxylic acid to capric acid for replicate injections is not more than 6.5%.
Procedure—
Separately inject equal volumes (about 0.2µL)of theStandard solution and theTest solution into the chromatograph,record the chromatograms,and measure the peak responses.Calculate the percentage of tetrahydro-2-furancarboxylic acid in the portion of Terazosin Hydrochloride taken by the formula:
100(C/W)(RU/RS),
in whichCis the concentration,in µg per mL,of tetrahydro-2-furancarboxylic acid in theStandard solution;Wis the weight,in mg,of Terazosin Hydrochloride taken to prepare theTest solution;andRUandRSare the peak response ratios obtained from theTest solution and theStandard solution,respectively:not more than 0.1%is found.
Limit of 1-[(tetrahydro-2-furanyl)carbonyl]piperazine—
Derivatization solution—
Dissolve about 2.0g of 3,5-dinitrobenzoyl chloride in 250mLof acetonitrile.
Phosphate buffer solution—
Transfer about 96.3g of dibasic potassium phosphate and 3.85g of monobasic potassium phosphate,each accurately weighed,to a 500-mLvolumetric flask.Dissolve in and dilute with water to volume.Adjust with phosphoric acid solution (10in 100)or sodium hydroxide solution (10in 100)to a pHof 8.0±0.1.Transfer 25.0mLof this solution to a 100-mLvolumetric flask,and dilute with water to volume.Adjust with phosphoric acid solution (10in 100)or sodium hydroxide solution (10in 100)to a pHof 8.0±0.1.
Solution A—
Use filtered and degassed water.
Solution B—
Use filtered and degassed acetonitrile.
Mobile phase—
Use variable mixtures ofSolution AandSolution Bas directed forChromatographic system.Make adjustments if necessary (seeSystem Suitability underChromatography á621ñ).
Blank solution—
Use acetonitrile.
Standard solution—
Dissolve an accurately weighed quantity of 1-[(tetrahydro-2-furanyl)carbonyl]piperazine in acetonitrile to obtain a solution having a known concentration of about 1.0mg per mL.Dilute quantitatively,and stepwise if necessary,with acetonitrile,to obtain a solution having a known concentration of about 5µg per mL.
Test solution—
Transfer about 125mg of Terazosin Hydrochloride,accurately weighed,to a 25-mLvolumetric flask;dissolve in and dilute with a mixture of acetonitrile and water (1:1)to volume;and mix.
Derivatization procedure—
Transfer 5-mLportions of theBlank solution,theStandard solution,and theTest solution,each to a separate 100-mLvolumetric flask,and proceed with each as follows.Add 5.0mLofPhosphate buffer solution,and mix.Add 10.0mLofDerivatization solution while swirling,allow to stand at room temperature for about 20minutes,and mix.Dilute with a mixture of acetonitrile and water (1:1)to volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm ×25-cm analytical column that contains packing L7.The flow rate is 1.5mLper minute,except it is changed to 2.0mLper minute during the period between 40and 80minutes.The chromatograph is programmed as follows.
Separately inject equal volumes (about 50µL)of the derivatizedBlank solution and the derivatizedStandard solution,and measure the peak responses as directed forProcedure,ensuring that the peaks in the chromatogram of the derivatizedStandard solution that correspond to those obtained from the derivatizedBlank solution do not interfere with the determination:the retention time for 1-[(tetrahydro-2-furanyl)carbonyl]piperazine is more than 22minutes;the column efficiency is not less than 3500theoretical plates;and the relative standard deviation for replicate injections is not more than 3.0%.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0–35 | 82 | 18 | isocratic |
| 35–40 | 82®10 | 18®90 | linear gradient |
| 40–75 | 10 | 90 | isocratic |
| 75–80 | 10®82 | 90®18 | linear gradient |
| 80–100 | 82 | 18 | isocratic |
Procedure—
Separately inject equal volumes (about 50µL)of the derivatizedStandard solution and the derivatizedTest solution into the chromatograph,record the chromatograms,and measure the peak areas.Calculate the percentage of 1-[(tetrahydro-2-furanyl)carbonyl]piperazine in the portion of Terazosin Hydrochloride taken by the formula:
2500(C/W)(rU/rS),
in whichCis the concentration,in mg per mL,of 1-[(tetrahydro-2-furanyl)carbonyl]piperazine in theStandard solution;Wis the weight,in mg,of Terazosin Hydrochloride taken to prepare theTest solution;andrUandrSare the peak areas for 1-[(tetrahydro-2-furanyl)carbonyl]piperazine derivative obtained from the derivatizedTest solution and the derivatizedStandard solution,respectively:not more than 0.1%is found.
Related compounds—
pH3.2Citrate buffer,Standard stock preparation,and Mobile phase—
Proceed as directed in the Assay.
Diluent 1—
Dissolve 6.0g of sodium citrate and 4.0g of anhydrous citric acid in water,dilute with water to 1.0L,and mix.
Diluent 2—
Prepare a mixture of water,acetonitrile,and methanol (60:30:10).
Standard stock solution 1—
Dissolve an accurately weighed quantity of USP Terazosin Related Compound A RSinDiluent 1,and dilute withDiluent 1to obtain a solution having a known concentration of about 0.5mg per mL.
Standard stock solution 2—
Dissolve an accurately weighed quantity of USP Terazosin Related Compound B RSin methanol,and dilute with methanol to obtain a solution having a known concentration of about 0.5mg per mL.
Standard stock solution 3—
Dissolve an accurately weighed quantity of USP Terazosin Related Compound C RSin Diluent 2,and dilute with Diluent 2to obtain a solution having a known concentration of about 0.1mg per mL.
Standard solution—
Transfer 5.0mLofStandard stock preparation,4.0mLofStandard stock solution 1,4.0mLofStandard stock solution 2,and 20mLofStandard stock solution 3to a 100-mLvolumetric flask containing about 60mLofDiluent 2.Dilute withDiluent 2to volume,and mix.Transfer 10.0mLof this solution to a 100-mLvolumetric flask,dilute withMobile phase to volume,and mix.
Test solution—
Use theAssay stock preparationprepared as directed in the Assay.
Chromatographic system—
Prepare as directed in theAssay.Chromatograph theMobile phase,and record the peak responses as directed forProcedure:ensure that there are no significant interfering peaks.Chromatograph theStandard solution,and record the peak responses as directed forProcedure:the relative retention times are about 0.2for terazosin related compound A,1.0for terazosin,1.48for terazosin related compound B,and 2.57for terazosin related compound C;the resolution,R,between terazosin and terazosin related compound Bis not less than 9.0;the column efficiency determined from the terazosin peak is not less than 12,000theoretical plates;the tailing factor for the terazosin related compound Cpeak is not more than 3.0;and the relative standard deviation for replicate injections determined from the terazosin peak is not more than 2.0%,and not more than 5.0%determined from the terazosin related compound Cpeak.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms for about 60minutes,and measure the peak responses.Separately calculate the quantities,in mg,of terazosin related compound Aand terazosin related compound Cin the portion of Terazosin Hydrochloride taken by the formula:
200C(rU/rS),
in whichCis the concentration,in mg per mL,of the appropriate USP Reference Standard in theStandard solution;andrUandrSare the peak responses for the corresponding related compound obtained from theTest solution and theStandard solution,respectively:not more than 0.3%of terazosin related compound Ais found;and not more than 0.4%of terazosin related compound Cis found.Calculate the quantity,in mg,of each impurity in the portion of Terazosin Hydrochloride taken by the formula:
200C(ri/rT),
in whichCis the concentration,in mg per mL,of USP Terazosin Hydrochloride RSin theStandard solution;riis the peak response for each impurity,other than terazosin related compound Aand terazosin related compound C,obtained from theTest solution;andrTis the terazosin peak response obtained from theStandard solution:not more than 0.3%of any impurity eluting prior to the terazosin peak is found;not more than 0.1%of any other impurity is found;and not more than 0.6%of total impurities is found.
Assay—
pH3.2Citrate buffer—
Dissolve 12.0g of sodium citrate dihydrate and 28.5g of anhydrous citric acid in 1.95Lof water.Adjust with anhydrous citric acid or sodium citrate to a pHof 3.2±0.1.Dilute with water to 2.0L,and mix.
Mobile phase—
Prepare a filtered and degassed mixture ofpH3.2Citrate buffer and acetonitrile (1685:315).Make adjustments if necessary (seeSystem Suitability underChromatography á621ñ).
Standard stock preparation—
Dissolve an accurately weighed quantity of USP Terazosin Hydrochloride RSinMobile phase,and dilute withMobile phase to obtain a solution having a known concentration of about 0.5mg per mL.
Standard preparation—
Transfer 10.0mLofStandard stock preparation to a 50-mLvolumetric flask,and dilute withMobile phase to volume.Transfer 10.0mLof this solution to a 100-mLvolumetric flask,dilute withMobile phase to volume;and mix.
Assay stock preparation—
Transfer about 100mg of Terazosin Hydrochloride,accurately weighed,to a 200-mLvolumetric flask;dissolve in and dilute withMobile phase to volume;and mix.
Assay preparation—
Transfer 10.0mLofAssay stock preparation to a 50-mLvolumetric flask,dilute withMobile phase to volume,and mix.Transfer l0.0mLof this solution to a l00-mLvolumetric flask,dilute withMobile phase to volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm ×25-cm column that contains packing L7.The column temperature is maintained at about 30 .The flow rate is about 1.0mLper minute.Chromatograph theMobile phase,and record the peak responses as directed forProcedure:ensure that there are no significant interfering peaks.Chromatograph theStandard preparation,and record the peak responses as directed forProcedure:the column efficiency is not less than 12,000theoretical plates;the tailing factor is not less than 0.9and not more than 1.3;and the relative standard deviation for replicate injections is not more than 0.9%.
.The flow rate is about 1.0mLper minute.Chromatograph theMobile phase,and record the peak responses as directed forProcedure:ensure that there are no significant interfering peaks.Chromatograph theStandard preparation,and record the peak responses as directed forProcedure:the column efficiency is not less than 12,000theoretical plates;the tailing factor is not less than 0.9and not more than 1.3;and the relative standard deviation for replicate injections is not more than 0.9%.
Procedure—
Separately inject equal volumes (about 20µL)of theStandard preparation and theAssay preparation into the chromatograph,record the chromatograms for about 45minutes,and measure the peak responses.Calculate the quantity,in mg,of C19H25N5O4·HCl in the portion of Terazosin Hydrochloride taken by the formula:
10,000C(rU/rS),
in whichCis the concentration,in mg per mL,of USP Terazosin Hydrochloride RSin theStandard preparation;andrUandrSare the peak responses obtained from theAssay preparation and theStandard preparation,respectively.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 1867
Pharmacopeial Forum:Volume No.29(5)Page 1580
Phone Number:1-301-816-8305