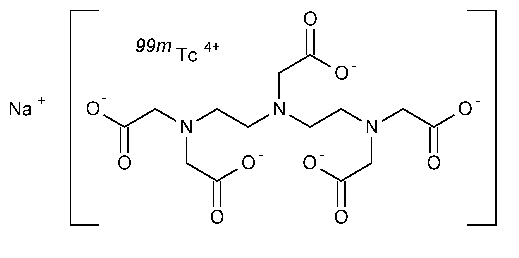
Technetium Tc 99m Pentetate Injection
Technetate(1-)99mTc,[N,N-bis[2-[bis(carboxymethyl)amino]ethyl]glycinato(5-)]-,sodium.
Sodium [N,N-bis[2-[bis(carboxymethyl)amino]ethyl]glycinato(5-)]-technetate(1-)-99mTc [65454-61-7].
»Technetium Tc 99m Pentetate Injection is a sterile solution of pentetic acid that is complexed with 99mTc in Sodium Chloride Injection.It is suitable for intravenous administration and may contain buffers.It contains not less than 90.0percent and not more than 110.0percent of the labeled amount of 99mTc as the pentetic acid complex expressed in megabecquerels (microcuries or millicuries)per mLat the time indicated in the labeling.Other chemical forms of radioactivity do not exceed 10.0percent of the total radioactivity.
Packaging and storage—
Preserve in single-dose or in multiple-dose containers,at a temperature between 2 and 8
and 8 .
.
Labeling—
Label it to include the following,in addition to the information specified for Labelingunder Injections á1ñ:the time and date of calibration;the amount of 99mTc as labeled pentetic acid complex expressed as total megabecquerels (millicuries or microcuries)and concentration as megabecquerels (microcuries or millicuries)per mLat the time of calibration;the expiration date;and the statement “Caution—Radioactive Material.”The labeling indicates that in making dosage calculations,correction is to be made for radioactive decay and also indicates that the radioactive half-life of 99mTc is 6.0hours.
Bacterial endotoxins á85ñ—
The limit of endotoxin content is not more than 175/VUSP Endotoxin Unit per mLof the Injection,when compared with the USP Endotoxin RS,in which Vis the maximum recommended total dose,in mL,at the expiration date or time.
pHá791ñ:
between 3.8and 7.5.
Radiochemical purity—
Phosphate buffer—
Dissolve 2.6g of monobasic sodium phosphate and 3.0g of anhydrous dibasic sodium phosphate in water to make 1000mL.Adjust,if necessary,by the addition of 0.2Mphosphoric acid or 0.2Msodium hydroxide,to a pHof 6.8±0.05.
Procedure
(see Electrophoresis á726ñ)—Soak a 2.5-×17.0-cm cellulose polyacetate strip in 100mLof Phosphate bufferfor 10to 60minutes.Remove the strip with forceps,taking care to handle the outer edges only.Place the strip between two absorbent pads,and blot to remove excess solution.Attach the strip to the support bridge of an electrophoresis chamber containing Phosphate bufferin each side of the chamber.Ensure that each end of the strip is in contact with Phosphate buffer.Mark the application line near the cathode end of the strip.Apply as a spot 1µLof a 1in 1000solution of amaranth in Phosphate buffer.Adjacent to this,apply as a narrow streak 5µLof Injection having an activity of about 1mCi per mL,previously diluted with saline TS,if necessary.Attach the chamber cover,and perform the electrophoresis at 300volts until the amaranth (red dye)has moved about four-fifths of the length of the strip.Remove the strip from the chamber,and blot the ends on paper towels.Using a suitable scanner and counting assembly,determine the radioactivity distribution.Labeled technetium pentetate migrates a distance that is 0.9relative to that of amaranth.Hydrolyzed technetium Tc 99m is located at the origin and pertechnetate migrates a distance that is 0.7relative to that of amaranth:the sum of the percentages of radioactivity of hydrolyzed technetium Tc 99m and pertechnetate is not greater than 10.0%of the total radioactivity on the strip.
Biological distribution—
Inject intravenously between 0.075MBq and 0.75MBq (2µCi and 20µCi)of Injection,in a volume not exceeding 0.2mL,into the caudal vein of each of three 20-to 25-g mice.Approximately 10to 15minutes after injection,place each animal in a suitable container and count the radioactivity of each with an appropriate radiation detector,using the same counting geometry,in order to determine the total activity injected.Approximately 24hours after injection,repeat the radioactivity counting of each animal,as above.After correcting for decay,determine the percentage of radioactivity retained in each animal 24hours after injection by the formula:
100(A/B),
in which Ais the net radioactivity,in counts per minute (corrected for decay),in the animal at 24hours,and Bis the total radioactivity,in net counts per minute,at the time of injection.The percentage of injected radioactivity retained 24hours after administration is not greater than 5.0%of the injected activity in any of the animals.
Other requirements—
It meets the requirements of the tests for Radionuclide identificationand Radionuclidic purityunder Sodium Pertechnetate Tc 99m Injection.It meets the requirements under Injections á1ñ,except that it may be distributed or dispensed prior to completion of the test for Sterility,the latter test being started on the day of manufacture,and except that it is not subject to the recommendation on Volume in Container.
Assay for radioactivity á821ñ—
Using a suitable counting assembly (see Selection of a Counting Assembly),determine the radioactivity,in MBq (µCi)per mL,of Injection by use of a calibrated system.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(RMI)Radiopharmaceuticals and Medical Imaging Agents
USP28–NF23Page 1860
Phone Number:1-301-816-8305