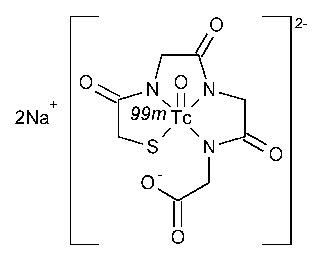
Technetium Tc 99m Mertiatide Injection
Technetate(2-)-99mTc,[N-[N-[N-(mercaptoacetyl)-glycyl]glycyl]glycinato(5-)-N,N¢,N¢¢,S]-oxo-,disodium,(SP-5-25)-.
Disodium [N-[N-[N-(mercaptoacetyl)glycyl]glycyl]glycinato(5-)-N,N¢,N¢¢,S]-oxo[99mTc]technetate(V) [125224-05-7].
»Technetium Tc 99m Mertiatide Injection is a sterile aqueous solution,suitable for intravenous injection,that contains 99mTc in the form of a chelate of mertiatide.It contains not less than 90.0percent and not more than 110.0percent of the labeled amount of 99mTc as mertiatide complex expressed in megabecquerels (or in microcuries or millicuries)per mLat the date and time indicated in the labeling.It contains uncomplexed betiatide,a suitable 99mTc reducing agent,a transfer ligand,and stabilizers.
Packaging and storage—
Preserve in single-dose or multiple-dose containers.
Labeling—
Label it to include the following,in addition to the information specified for Labelingunder Injections á1ñ:the time and date of calibration;the amount of 99mTc as labeled mertiatide expressed as total megabecquerels (or millicuries)and the concentration as megabecquerels per mL(or as millicuries per mL)on the date and time of calibration;the expiration date and time;and a statement,“Caution—Radioactive Material.”The labeling indicates that in making dosage calculations,correction is to be made for radioactive decay,and also indicates that the radioactive half-life of 99mTc is 6.0hours.
Bacterial endotoxins á85ñ—
It contains not more than 175/VUSP Endotoxin Unit per mL,in which Vis the maximum recommended total dose,in mL,at the expiration date or time.
pHá791ñ:
between 5.0and 6.0.
Radiochemical purity—
Method 1
(Determination of hydrolyzed reduced technetium)—Apply about 5to 10µL(100to 250µCi)15mm from the bottom of a 25-mm ×20-cm strip of chromatographic paper (see Chromatography á621ñ).Immediately develop the chromatogram by ascending chromatography using a solvent system consisting of a mixture of acetonitrile and water (60:40)until the solvent front has moved about 13cm from the origin.Remove the strip,and allow to dry.Determine the radioactivity distribution by scanning the chromatogram using a suitable collimated radiation detector.Calculate the percentage of hydrolyzed reduced technetium by the formula:
100(Aht/Bs),
in which Ahtis the sum of all the peaks at or near the origin,where RFis less than 0.25,and Bsis the sum of all of the peaks:not more than 2.0%of hydrolyzed reduced technetium is found.
Method 2
(Simultaneous determination of free pertechnetate and 99mTc mertiatide)—
SOLUTION A
—Prepare a filtered and degassed solution of monobasic potassium phosphate in water to obtain a solution containing 1.36mg per mL.To each liter of this solution,add 1.0mLof triethylamine,and adjust with 1.0Nhydrochloric acid to a pHof between 4.9and 5.1.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
SOLUTION B
—Prepare a filtered and degassed solution of monobasic potassium phosphate in 900mLof water,and add 100mLof tetrahydrofuran to obtain a solution containing 1.36mg per mL.To each liter of this solution,add 1.0mLof triethylamine,and adjust with 1.0Nhydrochloric acid to a pHof between 4.9and 5.1.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
MOBILE PHASE
—Mix specified portions of freshly prepared and degassed Solution Aand Solution B.
TEST SOLUTION
—Immediately before testing,dilute a portion of the Injection with Water for Injectionto obtain a concentration of between 400to 600µCi.[NOTE—The extent to which the sample is diluted is determined by the sensitivity of the radiometric detector.]
CHROMATOGRAPHIC SYSTEM
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 3.9-mm ×15-cm column that contains packing L1and a flow-through gamma-ray detector and is programmed to provide a Mobile phaseconsisting of variable mixtures of Solution Aand Solution B.The system is equilibrated for 15minutes with a Mobile phaseconsisting of a mixture of 90%Solution Aand 10%Solution B.After injecting the Test solution,change the composition of the Mobile phaselinearly over the next 30minutes so that the final Mobile phasecomposition consists of 20%Solution Aand 80%Solution B.Hold with this composition for 5minutes and then change in reverse to a composition of 90%Solution Aand 10%Solution Bover a 5-minute period.The flow rate is about 1mLper minute.
PROCEDURE
—Inject about 20µLof the Test solutioninto the chromatograph,record the chromatograms by gradient elution (see the Chromatographic system).The retention time is between 1.8and 2.2minutes for 99mTc pertechnetate and between 10and 14minutes for 99mTc mertiatide.Calculate the percentage of 99mTc pertechnetate by the formula:
100(rpt/rs),
in which rptis the peak response of 99mTc pertechnetate,and rsis the sum of all peak responses:not more than 6.0%of 99mTc pertechnetate is found.
Calculate the percentage of 99mTc mertiatide by the formula:
100(rmt/rs¢),
in which rmtis the peak response of 99mTc mertiatide,and rs¢is the sum of all peak responses:not less than 90.0%of 99mTc mertiatide is found.
Other requirements—
It meets the requirements of the tests for Radionuclide identificationand Radionuclidic purityunder Sodium Pertechnetate Tc 99m Injection.It also meets the requirement under Injections á1ñ,except that it may be distributed or dispensed prior to completion of the test for Sterility á71ñ,the latter test being started on the day of final manufacture,and except that it is not subject to the recommendation on Volume in Container.
Assay for radioactivity á821ñ—
Using a suitable counting assembly,determine the radioactivity,in Mbq (or millicuries)per mL,of the Injection by use of a calibrated system.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(RMI)Radiopharmaceuticals and Medical Imaging Agents
USP28–NF23Page 1858
Phone Number:1-301-816-8305