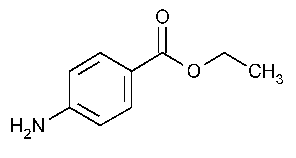
Benzocaine
»Benzocaine,dried over phosphorus pentoxide for 3hours,contains not less than 98.0percent and not more than 101.0percent of C9H11NO2.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
Infrared Absorption á197Kñ:previously dried over phosphorus pentoxide for 3hours.
B:
Ultraviolet Absorption á197Uñ—
Solution:
5µg per mL.
Medium:
chloroform.
Absorptivities at 278nm,calculated on the dried basis,do not differ by more than 3.0%.
C:
Dissolve about 20mg in 10mLof water with the aid of a few drops of 3Nhydrochloric acid,and add 5drops of a solution of sodium nitrite (1in 10),followed by 2mLof a solution of 100mg of 2-naphthol in 5mLof 1Nsodium hydroxide:an orange-red precipitate is formed.
Melting range,Class Iá741ñ:
between 88 and 92
and 92 ,but the range between beginning and end of melting does not exceed 2
,but the range between beginning and end of melting does not exceed 2 .
.
Reaction—
Dissolve 1.0g in 10mLof neutralized alcohol:a clear solution results.Dilute this solution with 10mLof water,and add 2drops of phenolphthalein TSand 1drop of 0.10Nsodium hydroxide:a red color is produced.
Loss on drying á731ñ—
Dry it over phosphorus pentoxide for 3hours:it loses not more than 1.0%of its weight.
Readily carbonizable substances á271ñ—
Dissolve 500mg in 5mLof sulfuric acid TS:the solution has no more color than Matching Fluid A.
Residue on ignition á281ñ:
not more than 0.1%.
Chloride—
To a solution of 200mg in 5mLof alcohol,previously acidified with a few drops of diluted nitric acid,add a few drops of silver nitrate TS:no turbidity is produced immediately.
Heavy metals,Method IIá231ñ:
0.001%.
Ordinary impurities á466ñ—
Test solution:
dehydrated alcohol.
Standard solution:
dehydrated alcohol.
Application volume:
10µL.
Eluant:
chloroform containing about 0.75%of dehydrated alcohol as a preservative,in a nonequilibrated chamber.
Visualization:
1.
Limit—
The total of any ordinary impurities observed does not exceed 1%.
Assay—
Dissolve about 300mg of Benzocaine,previously dried and accurately weighed,in a mixture of 100mLof water and 15mLof hydrochloric acid.Cool the solution in an ice bath to about 10 ,and titrate with 0.1Msodium nitrite VSuntil a blue color is produced immediately when the titrated solution is applied on starch iodide paper.When the titration is complete,the endpoint is reproducible after the mixture has been allowed to stand for 5minutes.Perform a blank determination,and make any necessary correction.Each mLof 0.1Msodium nitrite is equivalent to 16.52mg of C9H11NO2.
,and titrate with 0.1Msodium nitrite VSuntil a blue color is produced immediately when the titrated solution is applied on starch iodide paper.When the titration is complete,the endpoint is reproducible after the mixture has been allowed to stand for 5minutes.Perform a blank determination,and make any necessary correction.Each mLof 0.1Msodium nitrite is equivalent to 16.52mg of C9H11NO2.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 228
Pharmacopeial Forum:Volume No.30(3)Page 809
Phone Number:1-301-816-8379