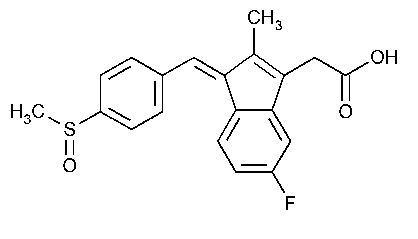
Sulindac
1H-Indene-3-acetic acid,5-fluoro-2-methyl-1-[[4-(methylsulfinyl)phenyl]methylene]-,(Z)-.
cis-5-Fluoro-2-methyl-1-[(p-methylsulfinyl)benzylidene]indene-3-acetic acid [38194-50-2].
»Sulindac contains not less than 99.0percent and not more than 101.0percent of C20H17FO3S,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
Infrared Absorption á197Mñ.
Solution:
15µg per mL.
Medium:
hydrochloric acid in methanol (1in 120).
Absorptivities at 284nm,calculated on the dried basis,do not differ by more than 3.0%.
Loss on drying á731ñ—
Dry it in vacuum at 100 for 2hours:it loses not more than 0.5%of its weight.
for 2hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.001%.
Chromatographic purity—
Standard preparation—
Prepare a solution of USP Sulindac RSin methanol having a concentration of 25mg per mL(Solution A).Prepare a second solution by diluting 1.0volume of Solution Awith methanol to obtain 250volumes of solution (Solution B).
Test preparation—
Prepare a solution of the sample in methanol having a concentration of 25mg per mL.
System suitability—
From the chromatograms obtained as directed under Procedure,estimate the intensity of the origin spot,if any,in the chromatogram of Solution A.The system is satisfactory if any spot observed at the origin is less intense than that obtained from the principal spot in the chromatogram of 2µLof Solution B.
Procedure—
Apply 4-µLportions of Solution Aand the Test preparationand 2-,4-,6-,8-,and 10-µLportions of Solution Bto a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Allow the spots to dry,and develop the chromatogram in a solvent system consisting of a mixture of ethyl acetate and glacial acetic acid (97:3)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,allow the solvent to evaporate,and examine the plate under short-wavelength UVlight:the chromatograms show principal spots at about the same RFvalue.Estimate the levels of any additional spots observed in the chromatogram of the Test preparationby comparison with the spots in the series of chromatograms of Solution B:the sum of the levels is not greater than that of the principal spot obtained from the 10-µLportion of Solution B(1%).
Organic volatile impurities,Method Vá467ñ:
meets the requirements except that the limit for chloroform is 500ppm.
Solvent—
Use dimethyl sulfoxide.
Assay—
Dissolve about 700mg of Sulindac,accurately weighed,in about 80mLof methanol,and titrate with 0.1Nsodium hydroxide VS,determining the endpoint potentiometrically,using a glass-calomel electrode system (see Titrimetry á541ñ).During the titration,and just prior to reaching the endpoint,wash down the walls of the titration vessel with small amounts of methanol.Each mLof 0.1Nsodium hydroxide is equivalent to 35.64mg of C20H17FO3S.
Auxiliary Information—
Staff Liaison:Daniel K.Bempong,Ph.D.,Scientist
Expert Committee:(PA2)Pharmaceutical Analysis 2
USP28–NF23Page 1838
Phone Number:1-301-816-8143