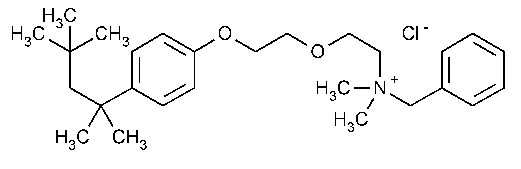
Benzethonium Chloride
Benzenemethanaminium,N,N-dimethyl-N-[2-[2-[4-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethyl]-,chloride.
Benzyldimethyl[2-[2-[p-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethyl]ammonium chloride [121-54-0].
»Benzethonium Chloride contains not less than 97.0percent and not more than 103.0percent of C27H42ClNO2,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:
To 1mLof a solution (1in 100)add 2mLof alcohol,0.5mLof 2Nnitric acid,and 1mLof silver nitrate TS:a white precipitate,which is insoluble in 2Nnitric acid but soluble in 6Nammonium hydroxide,is formed.
B:
Asolution (1in 100)forms precipitates with 2Nnitric acid and with mercuric chloride TS,both of which dissolve upon the addition of alcohol.
C:
Dissolve 0.1g in 1mLof sulfuric acid,add 0.1g of potassium nitrate,and heat on a steam bath for 3minutes.Cautiously dilute the solution with water to 10mL,add 0.5g of granulated zinc,and warm the mixture for 10minutes.Cool,add 0.2g of sodium nitrite to 1mLof the clear liquid,and add this mixture to 20mg of naphthol dipotassium disulfonate or naphthol disodium disulfonate in 1mLof ammonium hydroxide:the solution turns orange-red,and a brown precipitate may be formed.
Loss on drying á731ñ—
Dry it at 105 for 4hours:it loses not more than 5.0%of its weight.
for 4hours:it loses not more than 5.0%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Limit of ammonium compounds—
To 5mLof a solution (1in 50)add 3mLof 1Nsodium hydroxide,and heat to boiling:the odor of ammonia is not perceptible.
Assay—
Dissolve about 0.3g of Benzethonium Chloride,accurately weighed,in 75mLof water contained in a glass-stoppered,250-mLflask.Add 0.4mLof bromophenol blue solution (1in 2000),10mLof chloroform,and 1mLof 1Nsodium hydroxide.Titrate with 0.02Msodium tetraphenylboron VSuntil the blue color disappears from the chloroform layer.Add the last portions of the sodium tetraphenylboron solution dropwise,agitating vigorously after each addition.Each mLof 0.02Msodium tetraphenylboron is equivalent to 8.962mg of C27H42ClNO2.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 227
Pharmacopeial Forum:Volume No.27(1)Page 1754
Phone Number:1-301-816-8394