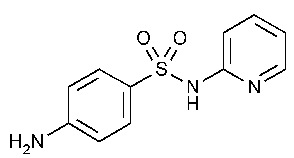
Sulfapyridine
»Sulfapyridine contains not less than 99.0percent and not more than 100.5percent of C11H11N3O2S,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed,light-resistant containers.
Clarity and color of solution—
Asolution of 1.0g in a mixture of 20mLof water and 5mLof 1Nsodium hydroxide is clear and not more deeply colored than pale yellow.
Identification—
A:
Infrared Absorption á197Kñ.
B:
Add 5mLof 3Nhydrochloric acid to about 0.1g of Sulfapyridine,and boil gently for about 5minutes.Cool in an ice bath,add 4mLof sodium nitrite solution (1in 100),dilute with water to 10mL,and place the mixture in the ice bath for 10minutes.To 5mLof the cooled mixture add a solution of 50mg of 2-naphthol in 2mLof sodium hydroxide solution (1in 10):an orange-red precipitate is formed,and it darkens on standing.
Acidity—
Digest 2.0g with 100mLof water at about 70 for 5minutes,cool at once to about 20
for 5minutes,cool at once to about 20 ,and filter.To 25.0mLof the filtrate add 2drops of phenolphthalein TS,and titrate with 0.10Nsodium hydroxide:not more than 0.20mLis required for neutralization.
,and filter.To 25.0mLof the filtrate add 2drops of phenolphthalein TS,and titrate with 0.10Nsodium hydroxide:not more than 0.20mLis required for neutralization.
Loss on drying á731ñ—
Dry it at 105 for 2hours:it loses not more than 0.5%of its weight.
for 2hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Selenium á291ñ:
0.003%,a 200-mg test specimen being used.
Heavy metals,Method IIá231ñ:
0.002%.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay—
Proceed with Sulfapyridine as directed under Nitrite Titration á451ñ.Each mLof 0.1Nsodium nitrite is equivalent to 24.93mg of C11H11N3O2S.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1832
Phone Number:1-301-816-8394