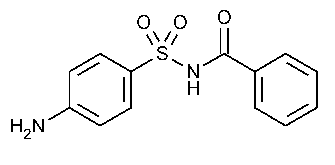
Sulfabenzamide
»Sulfabenzamide contains not less than 99.0percent and not more than 100.5percent of C13H12N2O3S,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed,light-resistant containers.
Clarity and color of solution—
Dissolve 2.0g in 15mLof 1Nsodium hydroxide,with warming:a colorless to pale yellow solution having not more than a slight turbidity is produced.
Identification—
A:
Infrared Absorption á197Kñ.
B:
To about 100mg,suspended in 2mLof water,add 100mg of sodium bicarbonate:it dissolves with effervescence (distinction from sulfanilamide,sulfapyridine,sulfathiazole,sulfadiazine,and sulfaguanidine).
Loss on drying á731ñ—
Dry it at 105 for 2hours:it loses not more than 0.5%of its weight.
for 2hours:it loses not more than 0.5%of its weight.
Selenium á291ñ:
0.001%,a 300-mg test specimen and 3mLof Stock Solutionbeing used.
Heavy metals,Method IIá231ñ:
0.002%.
Ordinary impurities á466ñ—
Test solution:
methanol.
Standard solution:
methanol.
Eluant:
a mixture of chloroform,methanol,and glacial acetic acid (90:5:5).
Visualization:
1.
Assay—
Transfer about 800mg of Sulfabenzamide,accurately weighed,to a 125-mLconical flask,and dissolve in 25mLof dimethylformamide.Add 3drops of thymol blue TS(prepared with methanol),and titrate with 0.1Nsodium methoxide VSto a blue endpoint.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nsodium methoxide is equivalent to 27.63mg of C13H12N2O3S.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1815
Phone Number:1-301-816-8394