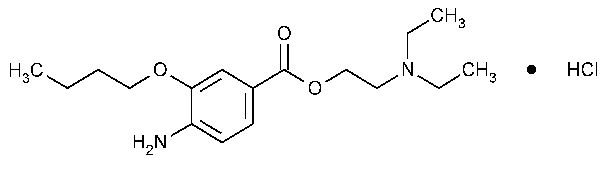
Benoxinate Hydrochloride
Benzoic acid,4-amino-3-butoxy-,2-(diethylamino)ethyl ester,monohydrochloride.
2-(Diethylamino)ethyl 4-amino-3-butoxybenzoate monohydrochloride [5987-82-6].
»Benoxinate Hydrochloride contains not less than 98.5percent and not more than 101.5percent of C17H28N2O3·HCl,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
Infrared Absorption á197Kñ:previously dried.
Solution:
15µg per mL.
Medium:
water.
C:
Asolution (1in 100)responds to the tests for Chloride á191ñ.
pHá791ñ:
between 5.0and 6.0,in a solution (1in 100).
Loss on drying á731ñ—
Dry it at 105 for 3hours:it loses not more than 1.0%of its weight.
for 3hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.2%.
Ordinary impurities á466ñ—
Test solution:
methanol.
Standard solution:
methanol.
Eluant:
a mixture of chloroform,cyclohexane,and diethylamine (5:4:1).
Visualization:
12.
Assay—
Dissolve about 250mg of Benoxinate Hydrochloride,accurately weighed,in a mixture of 20mLof glacial acetic acid and 20mLof acetic anhydride,and titrate with 0.1Nperchloric acid VS,determining the endpoint potentiometrically.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 34.49mg of C17H28N2O3·HCl.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 226
Phone Number:1-301-816-8379