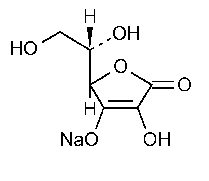
Sodium Ascorbate
»Sodium Ascorbate contains not less than 99.0percent and not more than 101.0percent of C6H7NaO6,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:
Infrared Absorption á197Mñ,on undried specimen.
B:
Add 1mLof 0.1Nhydrochloric acid to 4mLof a solution (1in 50):the solution reduces alkaline cupric tartrate TSslowly at room temperature but more readily upon heating.
C:
Asolution (1in 50)responds to the tests for Sodium á191ñ.
Specific rotation á781Sñ:
between +103 and +108
and +108 ,determined immediately following the preparation of the solution.
,determined immediately following the preparation of the solution.
Test solution:
100mg per mL,in carbon dioxide-free water.
pHá791ñ:
between 7.0and 8.0,in a solution (1in 10).
Loss on drying á731ñ—
Dry it in a suitable vacuum drying tube,phosphorus pentoxide being used as the desiccant,at 60 for 4hours:it loses not more than 0.25%of its weight.
for 4hours:it loses not more than 0.25%of its weight.
Heavy metals,Method IIá231ñ:
0.002%.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Dissolve about 400mg of Sodium Ascorbate,accurately weighed,in a mixture of 100mLof carbon dioxide-free water and 25mLof 2Nsulfuric acid.Titrate immediately with 0.1Niodine VS,adding 3mLof starch TSas the endpoint is approached.Each mLof 0.1Niodine is equivalent to 9.905mg of C6H7NaO6.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(DSN)Dietary Supplements:Non-Botanicals
USP28–NF23Page 1775
Phone Number:1-301-816-8389