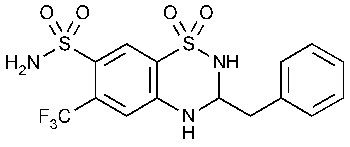
Bendroflumethiazide
2H-1,2,4-Benzothiadiazine-7-sulfonamide,3,4-dihydro-3-(phenylmethyl)-6-(trifluoromethyl)-,1,1-dioxide,(±)-.
(±)-3-Benzyl-3,4-dihydro-6-(trifluoromethyl)-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide [73-48-3].
»Bendroflumethiazide contains not less than 98.0percent and not more than 102.0percent of C15H14F3N3O4S2,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
USP Reference standards á11ñ—
USP Bendroflumethiazide RS.USP2,4-Disulfamyl-5-trifluoromethylaniline RS.
Identification—
A:
Infrared Absorption á197Kñ:previously dried over silica gel for 4hours.
B:
Ultraviolet Absorption á197Uñ—
Solution:
10µg per mL.
Medium:
methanol.
Absorptivities at 271nm,calculated on the anhydrous basis,do not differ by more than 4.0%.
C:
Mix 5mLof dilute hydrochloric acid (1in 2)with 20mg of Bendroflumethiazide,boil gently for 1minute,and cool in an ice bath.Add in succession 0.5mLof sodium nitrite solution (1in 1000),0.5mLof ammonium sulfamate solution (1in 200),and 0.5mLof N-(1-naphthyl)ethylenediamine dihydrochloride solution (1in 1000):a deep red color is produced.
Water,Method Iá921ñ:
not more than 0.5%.
Residue on ignition á281ñ:
not more than 0.2%.
Heavy metals,Method IIá231ñ:
0.002%.
Selenium á291ñ—
The absorbance from the Test Solutionprepared with 100mg of Bendroflumethiazide and 100mg of magnesium oxide,is not greater than one-half that from the Standard Solution(0.003%).
Limit of 2,4-disulfamyl-5-trifluoromethylaniline—
[NOTE—Use low-actinic glassware for the Test preparation and the Standard preparation.]
Mobile phase—
Dissolve 5.62g of sodium chloride and 1.97g of anhydrous sodium acetate in 1000mLof water in a 2-liter volumetric flask.Add 4.0mLof glacial acetic acid and 800mLof methanol,dilute with water to volume,mix,filter,and degas.
Standard preparation—
Dissolve an accurately weighed quantity of USP2,4-Disulfamyl-5-trifluoromethylaniline RSin methanol to obtain a solution having a known concentration of about 0.75µg per mL.
Test preparation—
Transfer about 25mg of Bendroflumethiazide,accurately weighed,to a 100-mLvolumetric flask,add methanol to volume,and mix.Transfer 10.0mLof this solution to a 50-mLvolumetric flask,dilute with methanol to volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 270-nm detector and a 4.6-mm ×30-cm column that contains packing L11maintained at a temperature of 35±5 .The flow rate is about 1.5mLper minute.Chromatograph five replicate injections of the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation is not more than 3.0%,and the resolution factor between the methanol and 2,4-disulfamyl-5-trifluoromethylaniline is not less than 1.4.
.The flow rate is about 1.5mLper minute.Chromatograph five replicate injections of the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation is not more than 3.0%,and the resolution factor between the methanol and 2,4-disulfamyl-5-trifluoromethylaniline is not less than 1.4.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Test preparationinto the chromatograph,record the chromatograms,and measure the peak response of the 2,4-disulfamyl-5-trifluoromethylaniline.Calculate the percentage of 2,4-disulfamyl-5-trifluoromethylaniline in the portion of Bendroflumethiazide taken by the formula:
100(CS/CU)(rU/rS),
in which CSis the concentration,in µg per mL,of USP2,4-Disulfamyl-5-trifluoromethylaniline RSin the Standard preparation;CUis the concentration,in µg per mL,of bendroflumethiazide in the Test preparation;and rUand rSare the peak responses obtained from the Test preparationand the Standard preparation,respectively.Not more than 1.5%of 2,4-disulfamyl-5-trifluoromethylaniline is found.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay—
Dissolve about 190mg of Bendroflumethiazide,accurately weighed,in 80mLof pyridine in a 250-mL,tall-form beaker in a well-ventilated hood.Add 3drops of a saturated solution of azo violet in methanol,cover the beaker,and gently bubble nitrogen through the solution for 5minutes,being careful to avoid any contact between the solution and the cover.Raise the nitrogen delivery tube above the solution surface and,maintaining a gentle flushing with nitrogen and stirring with a magnetic or mechanical stirring device,add 0.1Nsodium methoxide VSfrom a 10-mLburet inserted through an opening in the cover.Titrate to a blue endpoint,approaching the endpoint at a rate of 1or 2drops per second.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nsodium methoxide is equivalent to 21.07mg of C15H14F3N3O4S2.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 225
Phone Number:1-301-816-8305