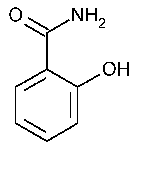
Salicylamide
»Salicylamide contains not less than 98.0percent and not more than 102.0percent of C7H7NO2,calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
Infrared Absorption á197Kñ.
Solution:
16µg per mL.
Medium:
methanol.
Absorptivities at 302nm,calculated on the anhydrous basis,do not differ by more than 3%.
C:
Dissolve about 100mg in 5mLof alcohol,and add a few drops of ferric chloride TS:a violet color develops.
Water,Method Iá921ñ:
not more than 0.5%.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.001%.
Chromatographic purity—
Standard preparations—
Dissolve USP Salicylamide RSquantitatively in methanol,and mix to obtain a solution having a concentration of 1.0mg per mL.Dilute quantitatively with methanol to obtain Standard preparations,designated by letter,having the following compositions:
| Standard preparation |
Dilution | Concentration (µg RS per mL) |
Percentage (%, for comparison with test specimen) |
| A
B C D |
(1in 5) (3in 20) (1in 10) (1in 20) |
200 150 100 50 |
1.0 0.75 0.5 0.25 |
Test preparation—
Dissolve 200mg of Salicylamide in 10.0mLof methanol,and mix.
Developing solvent system—
Prepare a mixture of normal butyl acetate,chloroform,and formic acid (6:4:2).
Procedure—
Apply separately 10µLof the Test preparationand 10µLof each of the Standard preparationsto a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture,and dry the spots with the aid of a current of air.Place the plate in a suitable chromatographic chamber,and develop the chromatograms with the Developing solvent systemuntil the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,allow the plate to dry,and locate the spots under short-wavelength UVlight.Compare the intensities of any secondary spots observed in the chromatogram of the Test preparationwith those of the principal spots in the chromatograms of the Standard preparations:the total of the intensities of all secondary spots obtained from the Test preparationdoes not exceed that of the principal spot obtained from Standard preparation B(1%).
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay—
Transfer about 500mg of Salicylamide,accurately weighed,to a 100-mLbeaker equipped with a mechanical stirrer and a suitable cover with a single hole for the buret tip.Add 30mLof freshly neutralized dimethylformamide containing a few drops of thymol blue TS.Titrate with 0.1Nsodium methoxide VSin toluene to the same blue endpoint obtained in the standardization of the sodium methoxide solution.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nsodium methoxide is equivalent to 13.71mg of C7H7NO2.
Auxiliary Information—
Staff Liaison:Daniel K.Bempong,Ph.D.,Scientist
Expert Committee:(PA2)Pharmaceutical Analysis 2
USP28–NF23Page 1746
Phone Number:1-301-816-8143