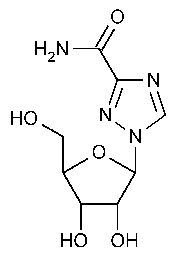
Ribavirin
»Ribavirin contains not less than 98.9percent and not more than 101.5percent of C8H12N4O5,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:Infrared Absorption á197Kñ.
B:Thin-Layer Chromatographic Identification Test á201ñ—
Test solution:
10mg per mL.
Developing solvent system:
a mixture of acetonitrile and 0.1Mammonium chloride (9:2).
Spray reagent—
Mix 0.5mLof anisaldehyde,0.5mLof sulfuric acid,0.1mLof glacial acetic acid,and 9mLof alcohol.
Procedure—
Proceed as directed in the chapter.Allow the plate to air-dry for about 15minutes,spray with Spray reagent,heat the plate at 110 for 30minutes,and locate the spots on the plate by examining the plate in daylight.
for 30minutes,and locate the spots on the plate by examining the plate in daylight.
Specific rotation á781Sñ:
between –33.5 and –37.0
and –37.0 (t=20
(t=20 ).
).
Test solution:
10mg per mL,in water.
pHá791ñ:
between 4.0and 6.5,in a solution (1in 50),to each 50mLof which has been added 0.2mLof a saturated potassium chloride solution.
Loss on drying á731ñ:
Dry it at 105 for 5hours:it loses not more than 0.5%of its weight.
for 5hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.25%.
Heavy metals,Method IIá231ñ:
0.001%.
Chromatographic purity—
Mobile phase,Standard preparation,Test solution,and Chromatographic system—
Prepare as directed in the Assay.
Procedure—
Inject about 10µLof the Test solutioninto the chromatograph,record the chromatogram,and measure the responses of all the peaks,except that of the solvent peak.Calculate the percentage of each peak,other than that of the solvent peak and the main ribavirin peak,by the formula:
100ri/rt,
in which riis the response of the individual peak,and rtis the sum of the responses of all the peaks in the chromatogram:not more than 0.25%of any individual peak is found,and the sum of all such peaks does not exceed 1.0%.
Assay—
Mobile phase—
Adjust water with sulfuric acid to a pHof 2.5±0.1.Filter through a suitable filter of 0.5-µm or finer porosity,and degas.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity of USP Ribavirin RSquantitatively in Mobile phaseto obtain a solution having a known concentration of about 0.025mg per mL.
Test solution—
Transfer about 50mg of Ribavirin,accurately weighed,to a 100-mLvolumetric flask,add about 50mLof Mobile phase,swirl to dissolve,dilute with Mobile phaseto volume,and mix.
Assay preparation—
Transfer 5.0mLof the Test solutionto a 100-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 207-nm detector and a 7.8-mm ×10-cm column that contains packing L17and is operated at 65±0.5 .The flow rate is about 1mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the tailing factor for the ribavirin peak is not less than 0.7and not more than 1.5,and the relative standard deviation for replicate injections is not more than 0.5%.
.The flow rate is about 1mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the tailing factor for the ribavirin peak is not less than 0.7and not more than 1.5,and the relative standard deviation for replicate injections is not more than 0.5%.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the peak area responses for the major peaks.Calculate the quantity,in mg,of C8H12N4O5in the portion of Ribavirin taken by the formula:
2000C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Ribavirin RSin the Standard preparation,and rUand rSare the ribavirin peak area responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1722
Pharmacopeial Forum:Volume No.27(3)Page 2577
Phone Number:1-301-816-8335