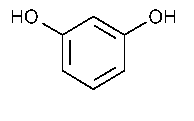
Resorcinol
»Resorcinol contains not less than 99.0percent and not more than 100.5percent of C6H6O2,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed,light-resistant containers.
Identification—
A:
Infrared Absorption á197Kñ—[NOTE—If necessary to recrystallize,dissolve in dehydrated alcohol.]
B:
Dissolve 100mg in 2mLof 1Nsodium hydroxide,add 1drop of chloroform,and heat the mixture:an intense crimson color is produced.Then add a slight excess of hydrochloric acid:the color changes to pale yellow.
C:
To 10mLof a solution (1in 100)add 1drop of ferric chloride TS:a blue-violet color is produced,and it fades slowly.
Loss on drying á731ñ—
Dry it over silica gel for 4hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.05%.
Phenol—
Heat gently a solution (1in 20):the odor of phenol is not perceptible.
Catechol—
To 10mLof a solution (1in 20)previously mixed with 2drops of 1Nacetic acid add 0.5mLof lead acetate TS:no turbidity is produced.
Ordinary impurities á466ñ—
Test solution:
methanol.
Standard solution:
methanol.
Eluant:
a mixture of hexanes and ethyl acetate (70:30).
Visualization:
17and then 1.
Application volume:
10µL.
Limits:
not more than 1.0%.
Organic volatile impurities,Method IVá467ñ:
meets the requirements.
Assay—
Dissolve about 1.5g of Resorcinol,accurately weighed,in water to make 500.0mL.Transfer to an iodine flask 25.0mLof the resulting solution,add 50.0mLof 0.1Nbromine VS,dilute with 50mLof water,add 5mLof hydrochloric acid,and at once insert the stopper in the flask.Shake for 1minute,allow to stand for 2minutes,and add 10mLof potassium iodide TSwhile slightly loosening the stopper.Shake thoroughly,allow to stand for 5minutes,remove the stopper,and rinse it and the neck of the flask with 20mLof water into the flask.Titrate the liberated iodine with 0.1Nsodium thiosulfate VS,adding starch TSas the endpoint is approached.Perform a blank determination.From the volume of 0.1Nsodium thiosulfate VSused,calculate the volume,in mL,of 0.1Nbromine VSconsumed by the resorcinol.Each mLof 0.1Nbromine is equivalent to 1.835mg of C6H6O2.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(PA6)Pharmaceutical Analysis 6
USP28–NF23Page 1720
Phone Number:1-301-816-8389