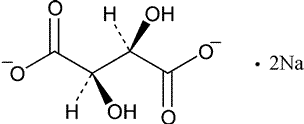
Add the following:
»Sodium Tartrate contains not less than 99.0percent and not more than 100.5percent of C4H4Na2O6·2H2O,calculated on the dried basis.
Identification—
A:
Responds to the tests for Sodium á191ñ.
B:
Responds to the tests for Tartrate á191ñ.
pHá791ñ:
between 7and 9(1in 10solution).
Loss on drying á731ñ—
Dry it at 150 for 3hours:it loses between 14.0%and 17.0%of its weight.
for 3hours:it loses between 14.0%and 17.0%of its weight.
Heavy metals,Method Iá231ñ:
0.002%.
Limit of oxalate—
Dissolve 1.0g of Sodium Tartrate in 10mLof water,then add 5drops of diluted acetic acid and 2mLof calcium chloride TS:no turbidity develops within 1hour.Not more than 0.1%is found.
Assay—
Transfer about 250mg of Sodium Tartrate,accurately weighed and previously dried at 150 for 3hours,to a 250-mLbeaker,and dissolve by stirring and heating to near the boiling point in 150mLof acetic acid.Cool the solution to room temperature.Titrate with 0.1Nperchloric acid VS(in glacial acetic acid),determining the endpoint potentiometrically.Perform a blank determination,and make any necessary adjustments (see Titrimetry á541ñ).Each mLof 0.1Nperchloric acid is equivalent to 9.703mg of C4H4Na2O6.
for 3hours,to a 250-mLbeaker,and dissolve by stirring and heating to near the boiling point in 150mLof acetic acid.Cool the solution to room temperature.Titrate with 0.1Nperchloric acid VS(in glacial acetic acid),determining the endpoint potentiometrically.Perform a blank determination,and make any necessary adjustments (see Titrimetry á541ñ).Each mLof 0.1Nperchloric acid is equivalent to 9.703mg of C4H4Na2O6. NF23
NF23
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(EMC)Excipients:Monograph Content
USP28–NF23Page 3082
Pharmacopeial Forum:Volume No.30(2)Page 611
Phone Number:1-301-816-8379