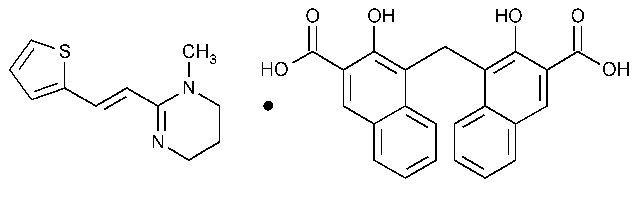
Pyrantel Pamoate
»Pyrantel Pamoate contains not less than 97.0percent and not more than 103.0percent of C34H30N2O6S,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed,light-resistant containers.
Identification—
A:Infrared Absorption á197Kñ.
C:
The chromatogram of the Assay preparationobtained as directed in the Assayexhibits major peaks due to pyrantel base and pamoic acid,the retention times of which correspond to those exhibited in the chromatogram of the Standard preparationobtained as directed in the Assay.
Loss on drying á731ñ—
Dry it in vacuum at 60 for 3hours:it loses not more than 2.0%of its weight.
for 3hours:it loses not more than 2.0%of its weight.
Residue on ignition á281ñ:
not more than 0.5%,from 1.33g.
Heavy metals,Method IIá231ñ:
0.005%.
Iron—
To the residue obtained in the test for Residue on ignitionadd 3mLof hydrochloric acid and 2mLof nitric acid,and evaporate on a steam bath to dryness.Dissolve the residue in 2mLof hydrochloric acid with the aid of gentle heat.Add 18mLof hydrochloric acid,dilute with water to 50mL,and mix.Dilute 5mLof this solution with water to 47mL:the limit is 0.0075%.
Related compounds—
TEST1—
Chromatographic sheet—
Impregnate 18-×56-cm filter paper (Whatman No.1or equivalent)with a freshly prepared 7:3mixture of acetone and glycine–sodium chloride–hydrochloric acid buffer solution (prepared by mixing 3volumes of a solution that is 0.3Mwith respect to both glycine and sodium chloride with 7volumes of 0.3Mhydrochloric acid).Press the impregnated paper uniformly between white,nonfluorescent blotters to remove the excess solvent.
Test solutions:
0.2and 20mg per mL,in a mixture of chloroform,methanol,and ammonium hydroxide (10:10:1).
Standard solutions:
0.2and 20mg per mL,in a mixture of chloroform,methanol,and ammonium hydroxide (10:10:1).
Application volume:
20µL.
Developing solvent system:
a mixture of ethyl acetate,butyl alcohol,and water (10:1:1).
Procedure—
Proceed as directed for Descending Chromatographyunder Chromatography á621ñ.Develop for 16to 20hours.Remove the sheet from the chamber,air-dry for 10minutes,transfer to an air-circulating oven,and dry at 60 for 30minutes.Examine the chromatogram on a 254-nm UVscanner screen:the RFvalue of the principal spot from the Test solutioncorresponds to that obtained from the appropriate Standard solution;and no spot in the chromatogram of the more concentrated Test solution,other than the principal spot,is larger or more intense than the principal spot from the less concentrated Test solution.
for 30minutes.Examine the chromatogram on a 254-nm UVscanner screen:the RFvalue of the principal spot from the Test solutioncorresponds to that obtained from the appropriate Standard solution;and no spot in the chromatogram of the more concentrated Test solution,other than the principal spot,is larger or more intense than the principal spot from the less concentrated Test solution.
TEST2—
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture.
Test stock solution—
Transfer about 100mg of Pyrantel Pamoate,accurately weighed,to a 10-mLvolumetric flask,dissolve in and dilute with dimethylformamide to volume,and mix.
Test solution—
Transfer 1.0mLof the Test stock solutionto a 100-mLvolumetric flask,dilute with dimethylformamide to volume,and mix.
Standard solution—
Transfer about 50mg of USP Pyrantel Pamoate RSto a 5-mLvolumetric flask,dilute with dimethylformamide to volume,and mix.
Developing solvent system:
a mixture of ethyl acetate,water,and glacial acetic acid (3:1:1).
Procedure—
Proceed as directed for Thin-Layer Chromatographyunder Chromatography á621ñ,except to line the developing chamber with filter paper,and allow to equilibrate.Apply 5-µLportions of the Test stock solution,the Test solution,and the Standard solutionto the plate,and allow to dry.Develop the chromatogram until the solvent front has moved about 10cm.Remove the plate from the developing chamber,and allow to air-dry for about 10minutes.Examine the plate under short-wavelength UVlight.The chromatograms obtained from the Test stock solutionand the Test solutionexhibit spots for pyrantel and the pamoate moiety at relative positions corresponding to those obtained from the chromatogram of the Standard solution:the RFvalue of pyrantel is about 0.3,and the RFvalue of the pamoate moiety is about 0.8.No spot obtained from the Test stock solution,other than that of pyrantel and the pamoate moiety,is more intense than the pyrantel spot obtained from the Test solution.
Content of pamoic acid—
Mobile phase and Chromatographic system—
Prepare as directed in the Assay.
Standard solution—
Dissolve an accurately weighed quantity of USP Pamoic Acid RSin Mobile phaseto obtain a solution having a known concentration of about 0.52mg per mL.Transfer 1.0mLof this solution to a 10-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Test solution—
Use the Assay preparation.
Procedure—
Inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and record the peak responses as directed in the Assay.Calculate the quantity,in mg,of C23H16O6in the portion of Pyrantel Pamoate taken by the formula:
1000C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Pamoic Acid RSin the Standard solutionand rUand rSare the peak responses for pamoic acid obtained from the Test solutionand the Standard solution,respectively:the content of pamoic acid is between 63.4%and 67.3%,calculated on the dried basis.
Assay—
[NOTE—Use low-actinic glassware in preparing solutions of pyrantel pamoate,and otherwise protect the solutions from unnecessary exposure to bright light.Complete the Assaywithout prolonged interruption.]
Mobile phase—
Prepare a mixture of acetonitrile,acetic acid,water,and diethylamine (92.8:3:3:1.2),filter,and degas.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).[NOTE—Increasing the amount of acetonitrile in Mobile phaseincreases retention times.Increasing the amount of acetic acid,water,and diethylamine decreases retention times.Should the Mobile phaseneed to be adjusted,maintain the ratios among acetic acid,water,and diethylamine (1:1:0.4).]
Standard preparation—
Prepare a solution in Mobile phasehaving an accurately known concentration of about 80µg of USP Pyrantel Pamoate RSper mL.
Assay preparation—
Transfer about 80mg of Pyrantel Pamoate,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with Mobile phaseto volume,and mix.Dilute 1.0mLof this solution with Mobile phaseto 10.0mL,and mix.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 288-nm detector and 4.6-mm ×25-cm column that contains packing L3.The flow rate is about 1.0mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the resolution,R,between pyrantel and pamoic acid is not less than 10.0;the number of theoretical plates for the pyrantel peak is not less than 8000;the tailing factor for the pyrantel peak is not greater than 1.3;and the relative standard deviation for replicate injections is not more than 1.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms obtained for a period of not less than 2.5times the retention times of pyrantel,and measure the responses for the major peaks.The relative retention times for pamoic acid and pyrantel are about 0.6and 1.0,respectively.Calculate the quantity,in mg,of C34H30N2O6Sin the portion of Pyrantel Pamoate taken by the formula:
1000C(rU/rS),
in which Cis the concentration,in mg,of USP Pyrantel Pamoate RSin the Standard preparation,and rUand rSare the peak responses for pyrantel obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1678
Pharmacopeial Forum:Volume No.27(3)Page 2576
Phone Number:1-301-816-8394