Prednisolone Sodium Succinate for Injection
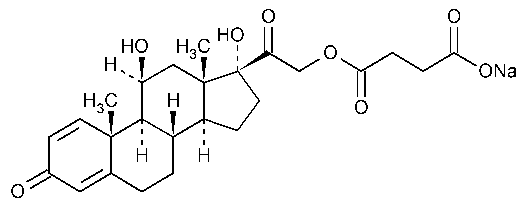
Pregna-1,4-diene-3,20-dione,21-(3-carboxy-1-oxopropoxy)-11,17-dihydroxy-,monosodium salt,(11b)-.
11b,17,21-Trihydroxypregna-1,4-diene-3,20-dione 21-(sodium succinate) [1715-33-9].
»Prednisolone Sodium Succinate for Injection is sterile prednisolone sodium succinate prepared from Prednisolone Hemisuccinate with the aid of Sodium Hydroxide or Sodium Carbonate.It contains the equivalent of not less than 90.0percent and not more than 110.0percent of the labeled amount of prednisolone (C21H28O5).It contains suitable buffers.
Constituted solution—
At the time of use,it meets the requirements for Constituted Solutionsunder Injections á1ñ.
Identification—
Place about 50mg in a separator,add 20mLof water and 2mLof 3Nhydrochloric acid,and extract with 25mLof chloroform.Filter the extract into a suitable beaker,evaporate on a steam bath to dryness,and dry the residue at 60 for 1hour:the residue so obtained responds to Identificationtest Aunder Prednisolone Hemisuccinate.
for 1hour:the residue so obtained responds to Identificationtest Aunder Prednisolone Hemisuccinate.
Bacterial endotoxins á85ñ—
It contains not more than 5.8USP Endotoxin Units per mg of prednisolone.
pHá791ñ:
between 6.7and 8.0,determined in the solution constituted as directed in the labeling.
Loss on drying á731ñ—
Dry it at 105 for 3hours:it loses not more than 2.0%of its weight.
for 3hours:it loses not more than 2.0%of its weight.
Particulate matter á788ñ:
meets the requirements under small-volume injections.
Other requirements—
It meets the requirements for Sterility Tests á71ñ,Uniformity of Dosage Units á905ñand Labelingunder Injections á1ñ.
Assay—
Standard preparation—
Dissolve about 64mg of USP Prednisolone Hemisuccinate RS,accurately weighed,in about 100mLof alcohol,add 5.0mLof water,dilute with alcohol to 200.0mL,and mix.Dilute 4.0mLof this solution with alcohol to 100.0mL,and mix.Pipet 20mLof the resulting solution into a glass-stoppered,50-mLconical flask.
Assay preparation—
Transfer an accurately weighed portion of Prednisolone Sodium Succinate for Injection,equivalent to about 50mg of prednisolone,to a 200-mLvolumetric flask,dissolve in 5.0mLof water,add alcohol to volume,and mix.Dilute 4.0mLof this mixture with alcohol to 100.0mL,and mix.Pipet 20mLof the resulting solution into a glass-stoppered,50-mLconical flask.
Procedure—
Proceed as directed under Assay for Steroids á351ñ.Calculate the quantity,in mg,of C21H28O5in the portion of Prednisolone Sodium Succinate for Injection taken by the formula:
5C(0.7827)(AU/AS),
in which Cis the concentration,in µg per mL,of USP Prednisolone Hemisuccinate RSin the Standard preparation,0.7827is the ratio of the molecular weight of prednisolone to that of prednisolone hemisuccinate,and AUand ASare the absorbances of the solutions from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 1613
Phone Number:1-301-816-8139