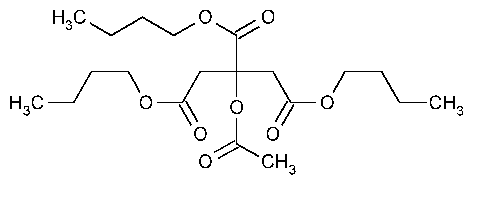
Acetyltributyl Citrate
»Acetyltributyl Citrate contains not less than 99.0percent of C20H34O8,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Identification—
B:
The retention time for the major peak in the chromatogram of theAssay preparation corresponds to that in the chromatogram of a similar preparation of USP Acetyltributyl Citrate RS.
Specific gravity á841ñ:
between 1.045and 1.055.
Refractive index á831ñ:
between 1.4410and 1.4425.
Acidity—
Dissolve 32.0g in 30mLof isopropyl alcohol,previously neutralized to bromothymol blue,add bromothymol blue TS,and titrate with 0.10Nsodium hydroxide to a faint blue endpoint:not more than 1.0mLis required.
Water,Method Iá921ñ:
not more than 0.25%.
Heavy metals,Method IIá231ñ:
0.001%.
Assay—
System suitability solution—
Prepare a solution in toluene containing about 30mg each of USP Acetyltributyl Citrate RSand USP Tributyl Citrate RSper mL.
Assay preparation—
Transfer about 300mg of Acetyltributyl Citrate,accurately weighed,to a 10-mLvolumetric flask,dissolve in and dilute with toluene to volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The gas chromatograph is equipped with an on-column,temperature-programmable injector,a flame-ionization detector maintained at about 275 ,and a 0.32-mm ×30-m column bonded with a 0.5-µm layer of phase G42.The column temperature is programmed to be maintained at about 80
,and a 0.32-mm ×30-m column bonded with a 0.5-µm layer of phase G42.The column temperature is programmed to be maintained at about 80 for 0.5minute,then to increase to about 220
for 0.5minute,then to increase to about 220 at a rate of 20
at a rate of 20 per minute,and to hold at about 220
per minute,and to hold at about 220 for 10minutes.The injection port temperature is programmed to be maintained at about 85
for 10minutes.The injection port temperature is programmed to be maintained at about 85 for 0.5minute,then to increase to about 225
for 0.5minute,then to increase to about 225 at a rate of 20
at a rate of 20 per minute,and to hold at about 225
per minute,and to hold at about 225 for 10minutes.Helium is used as the carrier gas at a flow rate of about 2.3mLper minute.Chromatograph theSystem suitability solution,and record the peak responses as directed forProcedure:the relative retention times are about 0.9for tributyl citrate and 1.0for acetyltributyl citrate;the resolution,R,between tributyl citrate and acetyltributyl citrate is not less than 1.5;and the relative standard deviation for replicate injections is not more than 2.0%determined from both the tributyl citrate and acetyltributyl citrate peaks,based on area percent calculation.
for 10minutes.Helium is used as the carrier gas at a flow rate of about 2.3mLper minute.Chromatograph theSystem suitability solution,and record the peak responses as directed forProcedure:the relative retention times are about 0.9for tributyl citrate and 1.0for acetyltributyl citrate;the resolution,R,between tributyl citrate and acetyltributyl citrate is not less than 1.5;and the relative standard deviation for replicate injections is not more than 2.0%determined from both the tributyl citrate and acetyltributyl citrate peaks,based on area percent calculation.
Procedure—
[NOTE—Use peak areas where peak responses are indicated.]Inject 1µLof the Assay preparationinto the chromatograph,record the chromatogram,and measure all of the peak areas,excluding the solvent peak.Calculate the percentage of C20H34O8in the portion of Acetyltributyl Citrate taken by the formula:
100(A/B),
in whichAis the acetyltributyl citrate peak response;andBis the sum of the responses of all the peaks.
Auxiliary Information—
Staff Liaison:Daniel K.Bempong,Ph.D.,Scientist
Expert Committee:(EMC)Excipients:Monograph Content
USP28–NF23Page 2949
Pharmacopeial Forum:Volume No.27(5)Page 3058
Phone Number:1-301-816-8143