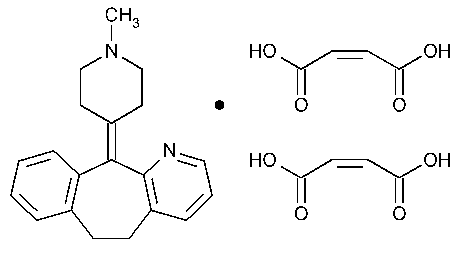
Azatadine Maleate
5H-Benzo[5,6]cyclohepta[1,2-b]pyridine,6,11-dihydro-11-(1-methyl-4-piperidinylidene)-,(Z)-2-butenedioate (1:2).
6,11-Dihydro-11-(1-methyl-4-piperidylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine maleate (1:2) [3978-86-7].
»Azatadine Maleate contains not less than 98.0percent and not more than 102.0percent of C20H22N2·2C4H4O4,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
Infrared Absorption á197Mñ.
Solution:
40µg per mL.
Medium:
0.25Nhydrochloric acid in methanol.
Loss on drying á731ñ—
Dry it in vacuum at 60 for 3hours:it loses not more than 1.0%of its weight.
for 3hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Chromatographic purity—
Dissolve an accurately weighed quantity in a solvent mixture consisting of toluene and methanol (1:1),to obtain a solution having a known concentration of about 7mg of the test specimen per mL.Similarly prepare a Standard solution of USP Azatadine Maleate RSin the same medium having a known concentration of about 7mg per mL.Apply 100-µLportions of the test solution and the Standard solution to a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Allow the spots to dry,and develop the chromatogram in a solvent system consisting of a mixture of toluene,isopropyl alcohol,and diethylamine (10:10:1),until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and allow the solvent to evaporate.Locate the spots on the plate by visualization under short-wavelength UVlight.Separately transfer the silica gel mixture containing the principal spot from each track to suitable stoppered centrifuge tubes.[NOTE—Take care to separate the principal spots from any adjacent spots.]Similarly transfer an equal amount of silica gel from a blank section of the plate to a separate,suitable stoppered centrifuge tube.To each of the three tubes add 15.0mLof a solvent mixture consisting of methanol and 0.5Nhydrochloric acid (4:1),shake vigorously for about 15minutes,and centrifuge.Concomitantly determine the absorbances of the supernatant test solution and the Standard solution in 1-cm cells at the wavelength of maximum absorbance at about 284nm,with a suitable spectrophotometer,using the solution obtained from the blank section of the plate as the blank.Calculate the chromatographic purity taken by the formula:
100(AU/AS)(CS/CU),
in which AUand ASare the absorbances of the test solution and the Standard solution,respectively;and CSand CUare the concentrations,in mg per mL,of the Standard solution and the test solution,respectively.The chromatographic purity is not less than 98.0%.
Assay—
Dissolve about 650mg of Azatadine Maleate,accurately weighed,in 50mLof glacial acetic acid,add 2drops of crystal violet TS,and titrate with 0.1Nperchloric acid VS.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 26.13mg of C20H22N2·2C4H4O4.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 205
Phone Number:1-301-816-8379