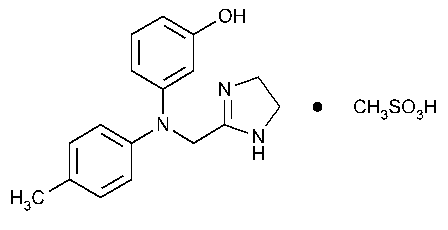
Phentolamine Mesylate
Phenol,3-[[(4,5-dihydro-1H-imidazol-2-yl)methyl](4-methylphenyl)amino]-,monomethanesulfonate (salt).
m-[N-(2-Imidazolin-2-ylmethyl)-p-toluidino]phenol monomethanesulfonate (salt) [65-28-1].
»Phentolamine Mesylate contains not less than 98.0percent and not more than 102.0percent of C17H19N3O·CH4O3S,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.Store at 25 ,excursions permitted between 15
,excursions permitted between 15 and 30
and 30 .
.
Identification—
A:Infrared Absorption á197Mñ.
C:
The RFvalue of the principal spot in the chromatogram of the Identification preparationcorresponds to that of Standard preparation Aas obtained in the test for Chromatographic purity.
Loss on drying á731ñ—
Dry it in vacuum at 60 for 4hours:it loses not more than 0.5%of its weight.
for 4hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Sulfate á221ñ—
A0.10-g portion shows no more sulfate than corresponds to 0.20mLof 0.020Nsulfuric acid (0.2%).
Chromatographic purity—
Standard preparations—
Dissolve USP Phentolamine Mesylate RSin methanol,and mix to obtain Standard preparation Ahaving a known concentration of 50µg per mL.Quantitatively dilute with methanol to obtain Standard preparations,designated below by letter,having the following compositions:
| Standard preparation |
Dilution | Concentration (µg RSper mL) |
Percentage (%, for comparison with test specimen) |
| A | (undiluted) | 50 | 0.5 |
| B | (3in 5) | 30 | 0.3 |
| C | (1in 5) | 10 | 0.1 |
Test preparation—
Dissolve an accurately weighed quantity of Phentolamine Mesylate in methanol to obtain a solution containing 10mg per mL.
Identification preparation—
Dilute a portion of the Test preparationquantitatively with methanol to obtain a solution containing 50µg per mL.
Detection reagent—
Prepare (1)a solution of 1g of potassium ferricyanide in 20mLof water,and (2)a solution of 1.9g of ferric chloride in 20mLof water.Just prior to use,mix equal volumes of the solutions.
Procedure—
Apply separately 5µLof the Test preparation,5µLof the Identification preparation,and 5µLof each Standard preparationto a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel,and allow to dry.Position the plate in a chromatographic chamber,and develop the chromatograms in a solvent system consisting of a mixture of chloroform,diethylamine,and methanol (15:3:2)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and dry the plate at 100 for 1hour.Spray the plate with Detection reagent.Within 15minutes after spraying,compare the intensities of any secondary spots observed in the chromatogram of the Test preparationwith those of the principal spots in the chromatograms of the Standard preparations:no secondary spot from the chromatogram of the Test preparationis larger or more intense than the principal spot obtained from Standard preparation A(0.5%),and the sum of the intensities of all secondary spots obtained from the Test preparationcorresponds to not more than 1.0%.
for 1hour.Spray the plate with Detection reagent.Within 15minutes after spraying,compare the intensities of any secondary spots observed in the chromatogram of the Test preparationwith those of the principal spots in the chromatograms of the Standard preparations:no secondary spot from the chromatogram of the Test preparationis larger or more intense than the principal spot obtained from Standard preparation A(0.5%),and the sum of the intensities of all secondary spots obtained from the Test preparationcorresponds to not more than 1.0%.
Assay—
0.1N Tetrabutylammonium hydroxide in isopropyl alcohol—
Dilute with dehydrated isopropyl alcohol a commercially available 25%solution of tetrabutylammonium hydroxide in methanol,and standardize as directed under Tetrabutylammonium Hydroxide,Tenth-Normal (0.1N)(see Volumetric Solutionsin the section Reagents,Indicators,and Solutions),using dehydrated isopropyl alcohol instead of dimethylformamide.
Procedure—
Dissolve with the aid of sonication,if necessary,about 300mg of Phentolamine Mesylate,accurately weighed,in 100mLof dehydrated isopropyl alcohol.Titrate in an atmosphere of nitrogen with 0.1N Tetrabutylammonium hydroxide in isopropyl alcohol,determining the endpoint potentiometrically,using a glass electrode and a calomel electrode containing a saturated solution of tetramethylammonium chloride in dehydrated isopropyl alcohol (see Titrimetry á541ñ).Perform a blank determination,and make any necessary correction.Each mLof 0.1Ntetrabutylammonium hydroxide is equivalent to 37.75mg of C17H19N3O·CH4O3S.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 1537
Pharmacopeial Forum:Volume No.29(5)Page 1562
Phone Number:1-301-816-8305