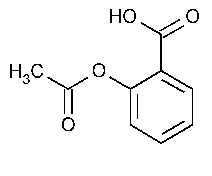
Aspirin
»Aspirin contains not less than 99.5percent and not more than 100.5percent of C9H8O4,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
Heat it with water for several minutes,cool,and add 1or 2drops of ferric chloride TS:a violet-red color is produced.
B:
Infrared Absorption á197Kñ.
Loss on drying á731ñ—
Dry it over silica gel for 5hours:it loses not more than 0.5%of its weight.
Readily carbonizable substances á271ñ—
Dissolve 500mg in 5mLof sulfuric acid TS:the solution has no more color than Matching Fluid Q.
Residue on ignition á281ñ:
not more than 0.05%.
Substances insoluble in sodium carbonate TS—
Asolution of 500mg in 10mLof warm sodium carbonate TSis clear.
Chloride á221ñ—
Boil 1.5g with 75mLof water for 5minutes,cool,add sufficient water to restore the original volume,and filter.A25-mLportion of the filtrate shows no more chloride than corresponds to 0.10mLof 0.020Nhydrochloric acid (0.014%).
Sulfate á211ñ—
Dissolve 6.0g in 37mLof acetone,and add 3mLof water.Titrate potentiometrically with 0.02Mlead perchlorate,prepared by dissolving 9.20g of lead perchlorate in water to make 1000mLof solution,using a pHmeter capable of a minimum reproducibility of ±0.1mV(see pHá791ñ)equipped with an electrode system consisting of a lead-specific electrode and a silver-silver chloride reference glass-sleeved electrode containing a 1in 44solution of tetraethylammonium perchlorate in glacial acetic acid (see Titrimetry á541ñ):not more than 1.25mLof 0.02Mlead perchlorate is consumed (0.04%).[NOTE—After use,rinse the lead-specific electrode with water,drain the reference electrode,flush with water,rinse with methanol,and allow to dry.]
Heavy metals—
Dissolve 2g in 25mLof acetone,and add 1mLof water.Add 1.2mLof thioacetamide-glycerin base TSand 2mLof pH3.5Acetate Buffer,and allow to stand for 5minutes:any color produced is not darker than that of a control made with 25mLof acetone and 2mLof Standard Lead Solution(see Heavy Metals á231ñ),treated in the same manner.The limit is 10µg per g.
Limit of free salicylic acid—
Dissolve 2.5g in sufficient alcohol to make 25.0mL.To each of two matched color-comparison tubes add 48mLof water and 1mLof a freshly prepared,diluted ferric ammonium sulfate solution (prepared by adding 1mLof 1Nhydrochloric acid to 2mLof ferric ammonium sulfate TSand diluting with water to 100mL).Into one tube pipet 1mLof a standard solution of salicylic acid in water,containing 0.10mg of salicylic acid per mL.Into the second tube pipet 1mLof the 1in 10solution of Aspirin.Mix the contents of each tube:after 30seconds,the color in the second tube is not more intense than that in the tube containing the salicylic acid (0.1%).
Organic volatile impurities,Method IVá467ñ:
meets the requirements.
Assay—
Place about 1.5g of Aspirin,accurately weighed,in a flask,add 50.0mLof 0.5Nsodium hydroxide VS,and boil the mixture gently for 10minutes.Add phenolphthalein TS,and titrate the excess sodium hydroxide with 0.5Nsulfuric acid VS.Perform a blank determination (see Residual Titrationsunder Titrimetry á541ñ).Each mLof 0.5Nsodium hydroxide is equivalent to 45.04mg of C9H8O4.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA2)Pharmaceutical Analysis 2
USP28–NF23Page 180
Pharmacopeial Forum:Volume No.30(4)Page 1164
Phone Number:1-301-816-8139