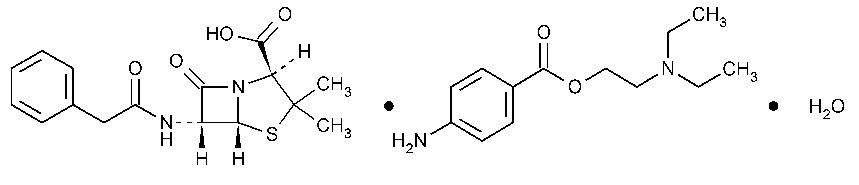
Penicillin G Procaine
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid,3,3-dimethyl-7-oxo-6-[(phenylacetyl)amino-],2S-(2a,5a,6b)-,compd.with 2-(diethylamino)ethyl 4-aminobenzoate (1:1)monohydrate.
(2S,5R,6R)-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with 2-(diethylamino)ethyl p-aminobenzoate (1:1)monohydrate [6130-64-9].
Anhydrous 570.71 [54-35-3].
»Penicillin G Procaine has a potency of not less than 900Penicillin G Units and not more than 1050Penicillin G Units per mg.
Labeling—
Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference standards á11ñ—
USP Penicillin G Potassium RS.USP Procaine Hydrochloride RS.USP Endotoxin RS.
Identification—
Prepare a solution of it containing about 12,000Penicillin G Units per mLin a solvent mixture consisting of acetone,0.1Mcitric acid,and 0.1Msodium citrate (2:1:1).Prepare a Standard solution of USP Penicillin G Potassium RScontaining about 12,000Penicillin G Units per mLin the same solvent mixture (Standard solution A).Prepare a Standard solution of USP Procaine Hydrochloride RScontaining about 5mg per mLin the same solvent system (Standard solution B).Apply separately 20µLof each solution to a thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Place the plate in a suitable chromatographic chamber,and develop the chromatogram in a solvent system consisting of a mixture of toluene,dioxane,and glacial acetic acid (90:25:4)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the chamber,mark the solvent front,and allow to air-dry.Examine the plate under short-and long-wavelength UVlight,noting the positions of the spots.Spray the plate with starch TSfollowed by dilute iodine TS(1in 10).Penicillin Gappears as a white spot on a purple background:the RFvalue of the penicillin Gspot obtained from the test solution corresponds to that obtained from Standard solution A.Spray the location of the spots visualized with UVlight with a 1in 20solution of p-dimethylaminobenzaldehyde in methanol.Procaine appears as a bright yellow spot:the RFvalue of the procaine spot obtained from the test solution corresponds to that obtained from Standard solution B.
Crystallinity á695ñ:
meets the requirements.
Bacterial endotoxins á85ñ—
Where the label states that Penicillin G Procaine is sterile or that it must be subjected to further processing during the preparation of injectable dosage forms it contains not more than 0.01USP Endotoxin Unit per 100Penicillin G Units.
Sterility á71ñ—
Where the label states that Penicillin G Procaine is sterile it meets the requirements when tested as directed for Membrane Filtrationunder Test for Sterility of the Product to be Examined,except to use Fluid Ato which has been added sufficient sterile penicillinase to inactivate the penicillin Gand to swirl the vessel until solution is complete before filtering.
pHá791ñ:
between 5.0and 7.5,in a (saturated)solution containing about 300mg per mL.
Water,Method Iá921ñ:
between 2.8%and 4.2%.
Content of Penicillin Gand procaine—
Mobile phase—
Dissolve 14g of monobasic potassium phosphate and 6.5g of tetrabutylammonium hydroxide solution (4in 10)in about 700mLof water,adjust with 1Npotassium hydroxide to a pHof 7.0,dilute with water to 1000mL,and mix.Mix 500mLof this solution,250mLof acetonitrile,and 250mLof water.Adjust with 1Npotassium hydroxide or dilute phosphoric acid (1in 10)to a pHof 7.5±0.05,filter through a membrane filter of 5µm or finer porosity,and degas.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Using accurately weighed quantities of USP Penicillin G Potassium RSand USP Procaine Hydrochloride RS,prepare a solution in Mobile phasehaving known concentrations of about 0.8mg per mLand 0.54mg per mL,respectively.
Test preparation—
Transfer about 70mg of Penicillin G Procaine,accurately weighed,to a 50-mLvolumetric flask,add about 30mLof Mobile phase,sonicate to dissolve,dilute with Mobile phaseto volume,and mix.
Resolution solution—
Prepare a solution of penicillin Vpotassium in Mobile phasecontaining 2.4mg per mL.Mix 1volume of this solution and 3volumes of Standard preparation.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 235-nm detector and a 4-mm ×30-cm column that contains 10-µm packing L1.The flow rate is about 1mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 3.0%.Chromatograph about 10µLof the Resolution solution,and record the peak responses as directed for Procedure:the resolution,R,between penicillin Gand penicillin Vis not less than 2.0.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Test preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.The relative retention times are 1.0for procaine and about 2.2for penicillin G.Calculate the percentage of penicillin G(C16H18N2O4S)in the specimen under test by the formula:
50C(GS/WU)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Penicillin G Potassium RSin the Standard preparation,GSis the designated penicillin Gcontent,in percentage,of USP Penicillin G Potassium RS,WUis the amount,in mg,of Penicillin G Procaine taken,and rUand rSare the responses of the penicillin Gpeaks obtained from the Test preparationand the Standard preparation,respectively:between 51.0%and 59.6%of C16H18N2O4Sis found.Calculate the percentage of procaine (C13H20N2O2)in the specimen under test by the formula:
(236.32/272.78)(5000C/WU)(rU/rS),
in which 236.32and 272.78are the molecular weights of procaine and procaine hydrochloride,respectively,Cis the concentration,in mg per mL,of USP Procaine Hydrochloride RSin the Standard preparation,WUis the amount,in mg,of Penicillin G Procaine taken,and rUand rSare the responses of the procaine peaks obtained from the Test preparationand the Standard preparation,respectively:between 37.5%and 43.0%is found.
Assay—
Standard preparation—
Using USP Penicillin G Potassium RS,prepare as directed for Standard preparationunderIodometric Assay—Antibiotics á425ñ.
Assay preparation—
Prepare as directed for Assay Preparationunder Iodometric Assay—Antibiotics á425ñ,except to dissolve about 100mg of Penicillin G Procaine,accurately weighed,in 2.0mLof methanol,and to dilute quantitatively with Buffer No.1to obtain a solution containing about 2000Penicillin G Units per mL.
Procedure—
Proceed as directed for Procedureunder Iodometric Assay—Antibiotics á425ñ.Calculate the potency,in Penicillin G Units per mg,of the Penicillin G Procaine taken by the formula:
F(B-I)/(2D),
in which Dis the concentration,in mg per mL,of the Assay preparation,on the basis of the weight of Penicillin G Procaine taken and the extent of dilution,and the other terms are as defined therein.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1489
Phone Number:1-301-816-8335