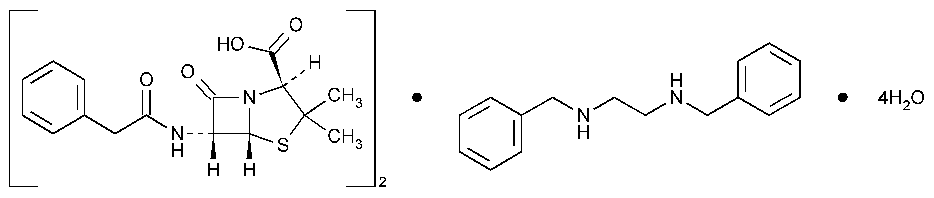
Penicillin G Benzathine
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid,3,3-dimethyl-7-oxo-6-[(phenylacetyl)amino-],2[S-(2a,5a,6b)]-,compd.with N,N¢-bis(phenylmethyl)-1,2-ethanediamine (2:1),tetrahydrate.
(2S,5R,6R)-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with N,N¢-dibenzylethylenediamine (2:1),tetrahydrate [41372-02-5].
Anhydrous 909.15 [1538-09-6].
»Penicillin G Benzathine has a potency of not less than 1090Penicillin G Units and not more than 1272Penicillin G Units per mg.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference standards á11ñ—
USP Endotoxin RS.USP Penicillin G Benzathine RS.USP Penicillin G Potassium RS.
Identification,
Ultraviolet Absorption á197Uñ—
Solution:
500µg per mL.
Medium:
methanol.
Absorptivity at 263nm is between 85.0%and 110.0%of that of USP Penicillin G Benzathine RS.
Crystallinity á695ñ:
meets the requirements.
Bacterial endotoxins á85ñ—
Where the label states that Penicillin G Benzathine is sterile or that it must be subjected to further processing during the preparation of injectable dosage forms it contains not more than 0.01USP Endotoxin Unit per 100Penicillin G Units.
Sterility á71ñ—
Where the label states that Penicillin G Benzathine is sterile it meets the requirements when tested as directed in the section Direct Inoculation of the Culture Mediumunder Test for Sterility of the Product to be Examined,except to use Fluid Thioglycollate Medium and Soybean–Casein Digest Medium containing polysorbate 80solution (1in 200)and an amount of sterile penicillinase sufficient to inactivate the penicillin Gin each tube,and to shake the vessels once daily.
pHá791ñ:
between 4.0and 6.5,in a solution prepared by dissolving 50mg in 50mLof dehydrated alcohol,adding 50mLof water,and mixing.
Water,Method Iá921ñ:
between 5.0%and 8.0%.
Benzathine content—
To about 1g of Pencillin G Benzathine,accurately weighed,add 30mLof a saturated solution of sodium chloride and 10mLof 5Nsodium hydroxide,and extract with four 50-mLportions of ether.Wash the combined ether extracts with three 10-mLportions of water.Extract the combined water washings with 25mLof ether,and add the ether extract to the water-washed combined ether extracts.Evaporate this combined ether solution to a volume of about 5mL,add 2mLof dehydrated alcohol,and evaporate to dryness.Dissolve the residue in 50mLof glacial acetic acid,add 1mLof p-naphtholbenzein TS,and titrate with 0.1Nperchloric acid VSto a green endpoint.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 12.02mg of benzathine (C16H20N2):between 24.0%and 27.0%of benzathine in Penicillin G Benzathine,calculated on the anhydrous basis,is found.
Assay—
0.05Mphosphate buffer,pH6.0—
Dissolve 6.8g of monobasic potassium phosphate in 900mLof water,adjust with 1Nsodium hydroxide to a pHof 6.0,dilute with water to 1000mL,and mix.
Mobile phase—
Prepare a mixture of 0.05Mphosphate buffer,pH6.0and acetonitrile (4:1),pass through a membrane filter having a 5-µm or finer porosity,and degas.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Transfer about 40mg of USP Penicillin G Potassium RS,accurately weighed,to a 50-mLvolumetric flask,add 10mLof acetonitrile and 5mLof methanol,and swirl to dissolve.Without delay,dilute with 0.05Mphosphate buffer,pH6.0to volume,and mix.
System suitability preparation—
Prepare a solution of penicillin Vpotassium in Mobile phasecontaining about 1mg per mL.Mix equal volumes of this solution and the Standard preparation.
Assay preparation—
Transfer about 53mg of Penicillin G Benzathine,accurately weighed,to a 50-mLvolumetric flask,add 10mLof acetonitrile and 5of methanol,and swirl to dissolve.Without delay,dilute with 0.05Mphosphate buffer,pH6.0to volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 225-nm detector and a 4-mm ×30-cm column that contains packing L1.The flow rate is about 2mLper minute.Chromatograph the Standard preparationand the System suitability preparation,and record the peak responses as directed for Procedure:the relative retention times are about 0.7for penicillin Gand 1.0for penicillin V;the resolution,R,between penicillin Gand penicillin Vis not less than 2.0;the column efficiency determined from the analyte peak is not less than 600theoretical plates;and the relative standard deviation for replicate injections of the Standard preparationis not more than 1.0%.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the potency,in Penicillin G Units per mg,of the Penicillin G Benzathine taken by the formula:
50(CP/W)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Penicillin G Potassium RSin the Standard preparation;Pis the stated potency,in Penicillin G Units per mg,of USP Penicillin G Potassium RS;Wis the quantity,in mg,of Penicillin G Benzathine taken to prepare the Assay preparation;and rUand rSare the penicillin Gpeak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1483
Phone Number:1-301-816-8335