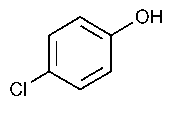
Parachlorophenol
»Parachlorophenol contains not less than 99.0percent and not more than 100.5percent of C6H5ClO.
Packaging and storage—
Preserve in tight,light-resistant containers.
Clarity and reaction of solution—
A1in 100solution is clear and is acid to litmus.
Identification—
A:
To a 1in 100solution of it add bromine TSdropwise:a white precipitate is formed,and at first it redissolves,but then it becomes permanent as an excess of the reagent is added.
B:
Add 1drop of ferric chloride TSto 10mLof a 1in 100solution of it:the solution acquires a violet-blue color.
C:
Heat a few crystals,held on a copper wire,in the edge of a nonluminous flame:a green color is imparted to the flame.
D:
To a mixture of 1g of it and 5mLof sodium hydroxide solution (1in 3)add 1.5g of monochloroacetic acid.Shake,and heat on a steam bath for 1hour.Cool,dilute with 15mLof water,and acidify with hydrochloric acid.Extract with 50mLof ether,wash the ether solution with 10mLof cold water,then extract the ether solution with 25mLof sodium carbonate solution (1in 20).Acidify the solution with hydrochloric acid,collect the resulting precipitate on a filter,and recrystallize it from hot water:the resulting parachlorophenoxyacetic acid melts between 154 and 158
and 158 .
.
Limit of nonvolatile residue—
Heat about 1g,accurately weighed,in a tared container on a steam bath until it is volatilized,and dry at 105 for 1hour:not more than 0.1%of residue remains.
for 1hour:not more than 0.1%of residue remains.
Chloride—
Acidify 10mLof a 1in 100solution with 2Nnitric acid,and add a few drops of silver nitrate TS:no turbidity or opalescence is produced.
Assay—
Transfer about 1g of Parachlorophenol,accurately weighed,to a 500-mLvolumetric flask,dissolve in and dilute with water to volume,and mix.Transfer a 25.0-mLportion of the solution to an iodine flask,cool in an ice bath to about 4 ,and add 20.0mLof 0.1Nbromine VS.Add 5mLof hydrochloric acid,and immediately insert the stopper.Maintain the flask at a temperature of 4
,and add 20.0mLof 0.1Nbromine VS.Add 5mLof hydrochloric acid,and immediately insert the stopper.Maintain the flask at a temperature of 4 for 30minutes,shaking at frequent intervals.Allow it to stand for 15minutes,remove the stopper just sufficiently to introduce quickly 5mLof potassium iodide solution (1in 5),taking care that no bromine vapor escapes,and at once insert the stopper in the flask.Shake thoroughly,remove the stopper,and rinse it and the neck of the flask with a small portion of water,allowing the washings to flow into the flask.Shake the mixture,and titrate the liberated iodine with 0.1Nsodium thiosulfate VS,using 3mLof starch TSas the indicator.Perform a blank determination (see Residual Titrationsunder Titrimetry á541ñ).Each mLof 0.1Nbromine is equivalent to 3.214mg of C6H5ClO.
for 30minutes,shaking at frequent intervals.Allow it to stand for 15minutes,remove the stopper just sufficiently to introduce quickly 5mLof potassium iodide solution (1in 5),taking care that no bromine vapor escapes,and at once insert the stopper in the flask.Shake thoroughly,remove the stopper,and rinse it and the neck of the flask with a small portion of water,allowing the washings to flow into the flask.Shake the mixture,and titrate the liberated iodine with 0.1Nsodium thiosulfate VS,using 3mLof starch TSas the indicator.Perform a blank determination (see Residual Titrationsunder Titrimetry á541ñ).Each mLof 0.1Nbromine is equivalent to 3.214mg of C6H5ClO.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1467
Phone Number:1-301-816-8394