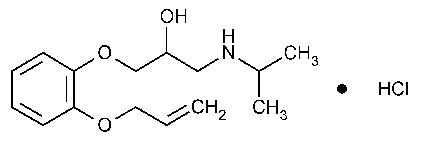
Oxprenolol Hydrochloride
2-Propanol,1-(o-allyloxyphenoxy)-3-isopropylamino-,hydrochloride.
1-(o-Allyloxyphenoxy)-3-isopropylamino-2-propanol hydrochloride [6452-73-9].
»Oxprenolol Hydrochloride contains not less than 98.5percent and not more than 101.0percent of C15H23NO3·HCl,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Clarity of solution—
Dissolve 1g in 10mLof water:solution is clear.
Identification—
A:
Infrared Absorption á197Kñ.
B:
Asolution of it responds to the tests for Chloride á191ñ.
pHá791ñ:
between 4.0and 6.0,in a solution (1in 10).
Loss on drying á731ñ—
Dry about 3g of it in vacuum at 60 for 6hours:it loses not more than 0.5%of its weight.
for 6hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.001%.
Chromatographic purity—
Diluting solvent—
Prepare a mixture of chloroform and dehydrated alcohol (1:1).
Standard solution A—
Prepare a solution of USP Oxprenolol Hydrochloride RSin Diluting solventcontaining 20mg per mL.
Standard solution B—
Dilute an accurately measured volume of Standard solution Aquantitatively,and stepwise if necessary,with Diluting solventto obtain a solution containing 0.08mg per mL.
Test solution—
Transfer 200mg of it to a 10-mLvolumetric flask,dissolve in Diluting solvent,dilute with Diluting solventto volume,and mix.
Procedure—
Apply separate 5-µLportions of the Test solutionand the Standard solutionsto the starting line of a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture,previously washed with methanol until the solvent front reaches the top of the plate,dried first in air and then at 100 for 20minutes,and cooled in a desiccator.Allow the spots to dry.Line a suitable chromatographic chamber with filter paper,saturate the paper with 100mLof a solvent system consisting of a mixture of ethyl acetate,glacial acetic acid,and water (15:5:5),and allow to stand for about 30minutes.Place the plate in the chamber,and develop the chromatogram until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the chamber and dry at 100
for 20minutes,and cooled in a desiccator.Allow the spots to dry.Line a suitable chromatographic chamber with filter paper,saturate the paper with 100mLof a solvent system consisting of a mixture of ethyl acetate,glacial acetic acid,and water (15:5:5),and allow to stand for about 30minutes.Place the plate in the chamber,and develop the chromatogram until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the chamber and dry at 100 for 15minutes.Spray the plate uniformly with a detection reagent consisting of a freshly prepared mixture of equal volumes of potassium ferricyanide solution (1in 100)and ferric chloride solution (1in 5).Dry the plate in a current of warm air for about 5minutes or until a spot from Standard solution Bis visible.Examine the chromatograms in ordinary light:the RFvalue of the principal spot from the Test solutioncorresponds to that obtained from Standard solution A.No spot other than the principal spot obtained from the Test solutionexceeds in size or intensity the principal spot obtained from Standard solution B(0.4%,corresponding to 0.2%of related compounds,the response factors for which are about double that of oxprenolol hydrochloride).
for 15minutes.Spray the plate uniformly with a detection reagent consisting of a freshly prepared mixture of equal volumes of potassium ferricyanide solution (1in 100)and ferric chloride solution (1in 5).Dry the plate in a current of warm air for about 5minutes or until a spot from Standard solution Bis visible.Examine the chromatograms in ordinary light:the RFvalue of the principal spot from the Test solutioncorresponds to that obtained from Standard solution A.No spot other than the principal spot obtained from the Test solutionexceeds in size or intensity the principal spot obtained from Standard solution B(0.4%,corresponding to 0.2%of related compounds,the response factors for which are about double that of oxprenolol hydrochloride).
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Dissolve about 450mg of Oxprenolol Hydrochloride in 100mLof glacial acetic acid.Add 10mLof mercuric acetate TS,and titrate with 0.1Nperchloric acid VS,determining the endpoint potentiometrically,using a glass-calomel electrode system (with a salt bridge of a saturated solution of lithium chloride in glacial acetic acid).Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 30.18mg of C15H23NO3·HCl.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 1430
Phone Number:1-301-816-8305