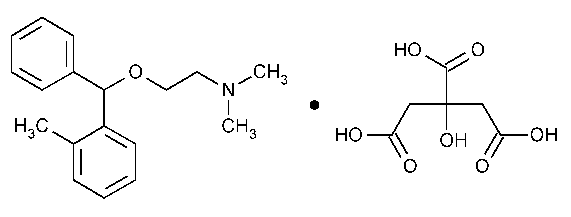
Orphenadrine Citrate
Ethanamine,N,N-dimethyl-2-[(2-methylphenyl)phenylmethoxy]-,(±)-,2-hydroxy-1,2,3-propanetricarboxylate (1:1).
(±)-N,N-Dimethyl-2-[(o-methyl-a-phenylbenzyl)oxy]ethylamine citrate (1:1) [4682-36-4].
»Orphenadrine Citrate contains not less than 98.0percent and not more than 101.5percent of C18H23NO·C6H8O7,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Clarity and color of solution—
Mix 1g of it with 10mLof a 1in 28solution of hydrochloric acid in alcohol:the solution is clear and its absorbance at 436nm is not greater than 0.050.
Identification—
A:
Infrared Absorption á197Mñ.
B:
Ultraviolet Absorption á197Uñ—
Solution:
500µg per mL.
Medium:
alcohol.
Absorptivities at 264nm,calculated on the dried basis,do not differ by more than 3.0%.
Loss on drying á731ñ—
Dry it at 105 for 3hours:it loses not more than 0.5%of its weight.
for 3hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Change to read:
Related compounds—
0.05M Ammonium phosphate buffer,Mobile phase,System sensitivity solution,and Chromatographic system—
Prepare as directed in theAssay.
Standard solution—
Use theStandard preparation,prepared as directed in theAssay.
Test solution—
Use theAssay preparation,prepared as directed in theAssay.
Procedure—
Separately inject equal volumes (about 20µL)of theTest solution and theStandard solution into the chromatograph,record the chromatogram for at least 2.5times the retention time of orphenadrine citrate,and measure all of the peak areas.Calculate the percentage of each impurity in the portion of Orphenadrine Citrate taken by the formula:
 in which Cis the concentration,in mg per mL,of USP Orphenadrine Citrate RSin the Standard solution;Wis the weight,in mg,of the sample taken to prepare the Test solution;Fis the relative response factor described in the table below;riis the peak area for each impurity in the Test solution;and rSis the peak area of Orphenadrine Citrate in the Standard solution:not more than 0.5%of total impurities is found.
in which Cis the concentration,in mg per mL,of USP Orphenadrine Citrate RSin the Standard solution;Wis the weight,in mg,of the sample taken to prepare the Test solution;Fis the relative response factor described in the table below;riis the peak area for each impurity in the Test solution;and rSis the peak area of Orphenadrine Citrate in the Standard solution:not more than 0.5%of total impurities is found.
 USP28
USP28
5000F(C/W)(ri/rS),
| Compound name | Relative Retention Time |
Relative Response Factor |
| Ethyldimethyl [2-(2-methylbenzhydryloxy)ethyl]ammonium chloride | 0.25 | 0.75 |
| 2-Methylbenzhydrol | 0.51 | 0.41 |
| Orphenadrine Citrate | 1.0 | — |
| N,N-Dimethyl-2-(o-tolyl-o-xyly loxy)ethylamine | 1.54 | 0.52 |
| Others | — | 1.0 |
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Isomer content—
Solvent—
Use carbon tetrachloride.
NMRreference—
Use tetramethylsilane.
Test preparation—
Place about 1g of Orphenadrine Citrate and 10mLof water in a 60-mLseparator,slowly add about 20drops of sodium hydroxide solution (1in 2),with swirling,to obtain a solution having a pHof about 10,and extract with three 15-mLportions of ether.Combine the ether extracts in a beaker,discarding the aqueous phase,and evaporate to about one-half the volume by warming on a steam bath under a stream of nitrogen.Transfer to a 60-mLseparator,wash with three 20-mLportions of water,and dry the ether solution with about 15g of anhydrous sodium sulfate in a 125-mLconical flask for 1hour,with intermittent swirling.Decant the dried ether solution through a pledget of glass wool into a small beaker.Rinse the sodium sulfate with two 10-mLportions of ether,and add the rinsings to the beaker.Evaporate most of the ether by warming under a stream of nitrogen,and remove the last traces of ether by drying at a pressure not exceeding 2mm of mercury at 60 .Transfer 400mg of the orphenadrine so obtained to a small weighing bottle,add 0.5mLof carbon tetrachloride and 1drop of tetramethylsilane,and swirl to dissolve.
.Transfer 400mg of the orphenadrine so obtained to a small weighing bottle,add 0.5mLof carbon tetrachloride and 1drop of tetramethylsilane,and swirl to dissolve.
Procedure—
Proceed as directed for Relative Method of Quantitationunder Nuclear Magnetic Resonance á761ñ,using the calculation formula given therein,in which A1is the sum of the average areas of the combined methine peaks associated with the meta-and para-methylbenzyl isomers,appearing at about 5.23ppm,and A2is the area of the methine peak associated with the ortho-methylbenzyl isomer,appearing at about 5.47ppm,with reference to the tetramethylsilane singlet at 0ppm,and both n1and n2are equal to 1:the limit of combined meta-and para-methylbenzyl isomers is 3.0%.
Assay—
0.05M Ammonium phosphate buffer—
Dissolve 5.8g of monobasic ammonium phosphate in 1000mLof water,and adjust with ammonium hydroxide or phosphoric acid to a pHof 7.9±0.05.
Mobile phase—
Prepare a filtered and degassed mixture of methanol,0.05M Ammonium phosphate buffer,and acetonitrile (9:8:3).Make adjustments if necessary (seeSystem Suitability underChromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity of USP Orphenadrine Citrate RSinMobile phase,and dilute quantitatively,and stepwise if necessary,withMobile phase to obtain a solution having a known concentration of about 0.9mg per mL.
System sensitivity solution—
Dilute a volume of theStandard preparationquantitatively,and stepwise if necessary,withMobile phase to obtain a solution having a known concentration of about 0.00045mg per mL.
Assay preparation—
Transfer about 45mg of Orphenadrine Citrate,accurately weighed,to a 50-mLvolumetric flask,dissolve in and dilute withMobile phase to volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm ×15-cm column that contains 5-µm packing L1.The flow rate is about 1.5mLper minute.The column temperature is maintained at 40 .Chromatograph theStandard preparation,and record the peak areas as directed forProcedure:the column efficiency is not less than 4500theoretical plates;the tailing factor is not more than 2.0;and the relative standard deviation for replicate injections is not more than 2.0%.Chromatograph theSystem sensitivity solution,and record the peak areas as directed forProcedure:the signal-to-noise ratio is not less than 10.
.Chromatograph theStandard preparation,and record the peak areas as directed forProcedure:the column efficiency is not less than 4500theoretical plates;the tailing factor is not more than 2.0;and the relative standard deviation for replicate injections is not more than 2.0%.Chromatograph theSystem sensitivity solution,and record the peak areas as directed forProcedure:the signal-to-noise ratio is not less than 10.
Procedure—
Separately inject equal volumes (about 20µL)of theStandard preparation and theAssay preparation into the chromatograph,record the chromatograms,and measure the peak areas for orphenadrine citrate.Calculate the quantity,in mg,of C18H23NO·C6H8O7in the portion of Orphenadrine Citrate taken by the formula:
50C(rU/rS),
in whichCis the concentration,in mg per mL,of USP Orphenadrine Citrate RSin theStandard preparation;andrUandrSare the peak responses obtained from theAssay preparation and theStandard preparation,respectively.
Auxiliary Information—
Staff Liaison:Ravi Ravichandran,Ph.D.,Senior Scientist
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 1422
Pharmacopeial Forum:Volume No.30(2)Page 523
Phone Number:1-301-816-8330