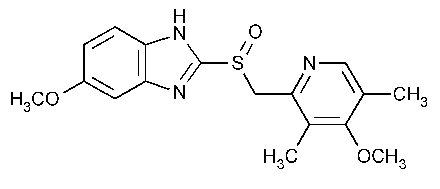
Omeprazole
1H-Benzimidazole,5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-.
5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]benzimidazole [73590-58-6].
»Omeprazole contains not less than 98.0percent and not more than 102.0percent of C17H19N3O3S,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers and store in a cold place,protected from moisture.
Identification—
A:
The RFvalue of the principal spot observed in the chromatogram of the Identification solutioncorresponds to that of the principal spot observed in the chromatogram of the Standard solutioncontaining 0.15mg of USP Omeprazole RSper mL,obtained as directed in the test for Chromatographic purity,Method 1.
B:
Infrared Absorption á197Kñ.
Completeness of solution á641ñ:
meets the requirements,a solution in methylene chloride containing 20mg per mLbeing used.
Color of solution—
Determine the absorbance of the solution prepared for the Completeness of solutiontest at 440nm,in 1-cm cells,using methylene chloride as the blank:the absorbance is not greater than 0.10.
Loss on drying á731ñ—
Dry it in vacuum at 60 for 4hours:it loses not more than 0.5%of its weight.
for 4hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.002%.
Organic volatile impurities,Method IVá467ñ:
meets the requirements.
Solvent—
Use dimethylacetamide.
Chromatographic purity—
METHOD1—
Solvent—
Prepare a mixture of dichloromethane and methanol (1:1).
Standard solutions—
Dissolve an accurately weighed quantity of USP Omeprazole RSin Solvent,and mix to obtain Standard solution Ahaving a known concentration of about 0.5mg per mL.Dilute this solution quantitatively with Solventto obtain Standard solution Band Standard solution Chaving known concentrations of about 0.15mg per mLand 0.05mg per mL,respectively.
Test solution—
Prepare a solution of Omeprazole in Solventcontaining 50mg per mL.
Identification solution—
Dilute a volume of the Test solutionquantitatively with Solventto obtain a solution containing 0.25mg per mL.
Procedure—
Separately apply 10µLof the Test solution,the Identification solution,and each of the Standard solutionsto a thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Allow the spots to dry,and develop the chromatogram in a solvent system consisting of a mixture of ammonia-saturated dichloromethane,dichloromethane,and isopropyl alcohol (2:2:1)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,allow the solvent to evaporate,and examine the plate under short-wavelength UVlight:the chromatograms show principal spots at about the same RFvalue.Estimate the intensities of any secondary spots observed in the chromatogram of the Test solutionby comparison with the spots in the chromatograms of the Standard solutions:no secondary spot from the chromatogram of the Test solutionis larger or more intense than the principal spot obtained from Standard solution B(0.3%),and the sum of the intensities of all secondary spots obtained from the Test solutionis no more intense than the principal spot obtained from Standard solution A(1.0%).
METHOD2—
Diluent—
Use Mobile phase.
Phosphate buffer,Mobile phase,System suitability solution,and Chromatographic system—
Proceed as directed in the Assay.
Test solution—
Dissolve an accurately weighed quantity of Omeprazole in Diluentto obtain a solution containing about 0.16mg per mL.[NOTE—Prepare this solution fresh.]
Procedure—
Inject equal volumes (about 40µL)of the Test solutionand Diluentinto the chromatograph,and allow the Test solutionto elute for not less than two times the retention time of omeprazole.Record the chromatograms,and measure the peak responses.Calculate the percentage of each impurity in the portion of Omeprazole taken by the formula:
100(ri/rs),
in which riis the peak response for each impurity,and rsis the sum of the responses of all of the peaks:not more than 0.3%of any individual impurity is found,and the sum of all impurities is not more than 1.0%.
Change to read:
Assay—
Phosphate buffer—
Dissolve 0.725g of monobasic sodium phosphate and 4.472g of anhydrous dibasic sodium phosphate in 300mLof water,dilute with water to 1000mL,and mix.Dilute 250mLof this solution with water to 1000mL. If necessary,adjust the pHwith phosphoric acid to 7.6.
If necessary,adjust the pHwith phosphoric acid to 7.6. USP28
USP28
Mobile phase—
Prepare a filtered and degassed mixture ofPhosphate buffer and acetonitrile (3:1).Make adjustments if necessary (seeSystem Suitability under Chromatography á621ñ).
Diluent—
Prepare a mixture of 0.01Msodium borate and acetonitrile (3:1).
Standard preparation—
Dissolve an accurately weighed quantity of USP Omeprazole RSinDiluent,and dilute quantitatively,and stepwise if necessary,withDiluent to obtain a solution having a known concentration of about 0.2mg per mL.
Assay preparation—
Transfer about 100mg of Omeprazole,accurately weighed,to a 50-mLvolumetric flask,dissolve in and dilute withDiluent to volume,and mix.Transfer 5.0mLof this solution to a 50-mLvolumetric flask,dilute withDiluent to volume,and mix.
System suitability solution—
Dilute a volume ofStandard preparation withDiluent to obtain a solution containing about 0.1mg of USP Omeprazole RSper mL.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 280-nm detector and a 4.6-mm ×15-cm column that contains 5-µm packing L7.The flow rate is about 0.8mLper minute.Chromatograph theSystem suitability solution,and record the peak responses as directed for Procedure:the capacity factor,k¢,is not less than 6.0;the column efficiency is not less than 3000theoretical plates;the tailing factor is not more than 1.5;and the relative standard deviation for replicate injections is not more than 1.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of C17H19N3O3Sin the portion of Omeprazole taken by the formula:
500C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Omeprazole RSin theStandard preparation;and rUand rSare the peak responses obtained from theAssay preparationand theStandard preparation,respectively.
Auxiliary Information—
Staff Liaison:Elena Gonikberg,Ph.D.,Scientist
Expert Committee:(PA4)Pharmaceutical Analysis 4
USP28–NF23Page 1416
Pharmacopeial Forum:Volume No.30(2)Page 522
Phone Number:1-301-816-8251