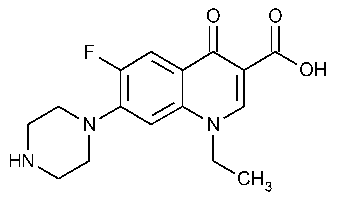
Norfloxacin
3-Quinolinecarboxylic acid,1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-.
1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid [70458-96-7].
»Norfloxacin contains not less than 99.0percent and not more than 101.0percent of C16H18FN3O3,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:
Infrared Absorption á197Mñ.
B:
Ultraviolet Absorption á197Uñ—[NOTE—Use-low actinic glassware in this procedure.]
Solution:
5µg per mL.
Medium:
0.1Nsodium hydroxide.
Absorptivities at 273nm,calculated on the dried basis,do not differ by more than 3.0%.
Loss on drying á731ñ—
Dry it in vacuum at a pressure not exceeding 5mm of mercury at 100 to constant weight:it loses not more than 1.0%of its weight.
to constant weight:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.1%,a platinum crucible being used.
Heavy metals,Method IIá231ñ:
0.0015%.
Chromatographic purity—
Dissolve a quantity of Norfloxacin in a mixture of methanol and methylene chloride (1:1)to obtain a test solution containing 8.0mg per mL.Dissolve 4.0mg of USP Norfloxacin RSin 1mLof glacial acetic acid,add 4mLof methanol,and mix.To 1mLof this Standard stock solution add 9mLof the mixture of methanol and methylene chloride (1:1)to obtain Comparison solution A.Dilute a portion of this solution with an equal volume of the mixture of methanol and methylene chloride (1:1)to obtain Comparison solution B.Separately apply 5µLof the test solution,1,1.5,and 2µLof Comparison solution A,and 5µLof Comparison solution Bto a suitable high-performance thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of silica gel mixture,previously washed with methanol and air-dried.The spots of Comparison solutions Aand Bare equivalent to 0.2,0.3,0.4,and 0.5%of impurities,respectively.Place the plate in a paper-lined chromatographic chamber previously equilibrated with a solvent system consisting of a mixture of chloroform,methanol,toluene,diethylamine,and water (40:40:20:14:8).Seal the chamber and allow the chromatogram to develop until the solvent front has moved about nine-tenths of the length of the plate.Remove the plate from the chamber,mark the solvent front,allow the solvent to evaporate,and examine the plate under both short-and long-wavelength UVlight.Compare the intensities of any secondary spots observed in the chromatogram of the test solution with those of the principal spots in the chromatograms of Comparison solutions Aand B:the sum of the intensities of secondary spots obtained from the test solution corresponds to not more than 0.5%of impurities.
Assay—
Dissolve about 460mg of Norfloxacin,accurately weighed,in 100mLof glacial acetic acid.Titrate potentiometrically with 0.1Nperchloric acid VSusing a suitable anhydrous electrode system (see Titrimetry á541ñ).[NOTE—Remove any aqueous solution in the electrode(s),render anhydrous,and fill with 0.1Nlithium perchlorate in acetic anhydride.]Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 31.93mg of C16H18FN3O3.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1399
Phone Number:1-301-816-8394