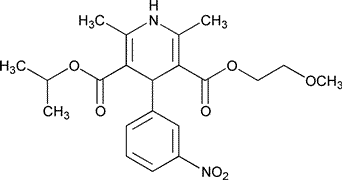
Nimodipine
»Nimodipine contains not less than 98.5percent and not more than 101.5percent of C21H26N2O7,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers,and store at 25 ,excursions permitted between 15
,excursions permitted between 15 and 30
and 30 .
.
USP Reference standards á11ñ—
USP Nimodipine RS.USP Nimodipine Related Compound A RS.
NOTE—Throughout the following procedures,protect test or assay specimens,the Reference Standards,and solutions containing them by conducting the procedures immediately under subdued light or using low-actinic glassware.
Identification—
B:
The retention time of the major peak in the chromatogram of the Test solutioncorresponds to that in the chromatogram of Standard solution 1,as obtained in the test for Chromatographic purity.
Loss on drying á731ñ—
Dry it at 105 for 4hours:it loses not more than 0.5%of its weight.
for 4hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Related compounds—
Mobile phase—
Prepare a filtered and degassed mixture of water,methanol,and tetrahydrofuran (3:1:1).
Standard solution 1—
Transfer about 40mg of USP Nimodipine RS,accurately weighed,to a 25-mLvolumetric flask,dissolve in 2.5mLof tetrahydrofuran,dilute with Mobile phaseto volume,and mix.Transfer 1.0mLof this solution to a 100-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.Transfer 2.0mLof this second solution to a 10-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Standard solution 2—
Transfer about 20.0mg of USP Nimodipine Related Compound A RS,accurately weighed,to a 25-mLvolumetric flask,dissolve in 2.5mLof tetrahydrofuran,dilute with Mobile phaseto volume,and mix.Transfer 5.0mLof this solution to a 100-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Standard solution 3—
Transfer 2.5mLof Standard solution 1to a 100-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Standard solution 4—
Transfer 1.0mLof Standard solution 2and 1.0mLof Standard solution 3to a 25-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Test solution—
Transfer about 40mg of Nimodipine,accurately weighed,to a 25-mLvolumetric flask,dissolve in 2.5mLof tetrahydrofuran,dilute with Mobile phaseto volume,and mix.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 235-nm detector and a 4.6-mm ×12.5-cm column that contains packing L1.The flow rate is about 2mLper minute.The column temperature is maintained at 40 .Chromatograph Standard solution 4,and record the peak responses as directed for Procedure:the relative retention times are about 0.9for nimodipine related compound Aand 1.0for nimodipine;the resolution,R,between nimodipine related compound Aand nimodipine is not less than 1.5;and the relative standard deviation for replicate injections is not more than 2.0%.
.Chromatograph Standard solution 4,and record the peak responses as directed for Procedure:the relative retention times are about 0.9for nimodipine related compound Aand 1.0for nimodipine;the resolution,R,between nimodipine related compound Aand nimodipine is not less than 1.5;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20µL)of Standard solution 1,Standard solution 4,and the Test solutioninto the chromatograph,record the chromatograms,and measure the peak responses.[NOTE—Record the chromatogram of the Test solutionfor a period of time equivalent to four times the retention time of nimodipine.]Calculate the percentage of each impurity in the portion of Nimodipine taken by the formula:
100C(ri/rS),
in which Cis the concentration,in mg per mL,of USP Nimodipine Related Compound A RSin Standard solution 4;riis the peak response of each impurity obtained from the Test solution;and rSis the peak response of nimodipine related compound Aobtained from Standard solution 4:not more than 0.1%of nimodipine related compound Ais found;not more than 0.2%of any other individual impurity is found;and not more than 0.5%of total impurities is found.
Assay—
Transfer about 180mg of Nimodipine,accurately weighed,to a 100-mLbeaker.Dissolve,with gentle heating,by stirring in a mixture of 25mLof tertiary butyl alcohol and 25mLof perchloric acid TS.Add 0.1mLof ferroin TS.Titrate with 0.1Nceric sulfate VS.Perform a blank determination,and make any necessary correction (see Titrimetry á541ñ).Each mLof 0.1Nceric sulfate is equivalent to 20.92mg of C21H26N2O7.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 1379
Pharmacopeial Forum:Volume No.29(4)Page 1057
Phone Number:1-301-816-8305