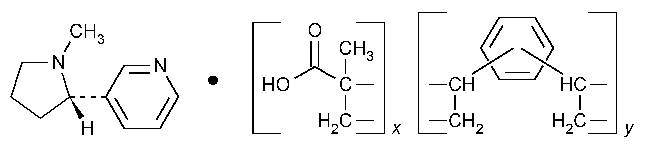
Nicotine Polacrilex
»Nicotine Polacrilex is a weak carboxylic cation-exchange resin prepared from methacrylic acid and divinylbenzene,in complex with nicotine.It contains not less than 95.0percent and not more than 115.0percent of the labeled amount of nicotine (C10H14N2),calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:Infrared Absorption á197Kñ—
Test specimen—
Transfer an accurately weighed quantity of Nicotine Polacrilex,equivalent to about 100mg of nicotine,to a 100-mLglass-stoppered tube.Add 20mLof 1Mammonium hydroxide,5mLof 10Msodium hydroxide,and 20mLof n-hexane.Shake for 5minutes,and allow the phases to separate.Transfer the upper hexane phase to an evaporating dish,and evaporate on a steam bath.
Standard specimen—
Use USP Nicotine Bitartrate Dihydrate RS,and proceed as directed for Test specimen.
B:Infrared Absorption á197Kñ—
Test specimen—
Use the residue obtained from the Assay preparation.Decant the ammonia solution remaining in each residue,and wash the residue by shaking it with three 10-mLvolumes of water,decanting the water phase after each shaking.Wash with 10mLof 0.1Nhydrochloric acid,decant the liquid,and dry the residue at about 105 .
.
Standard specimen—
Transfer an accurately weighed portion of USP Polacrilex Resin RS,equivalent to the amount of nicotine polacrilex in the Assay preparation,to a glass-stoppered tube.Add 10mLof 1Mammonium hydroxide.Proceed as directed for Test specimen,beginning with “Decant the ammonia”.
Nicotine release—
Transfer an accurately weighed quantity of Nicotine Polacrilex,equivalent to about 4mg of nicotine,to a glass-stoppered test tube,add 10.0mLof sodium chloride solution (0.9in 100)that has been warmed to 37 ,and shake by mechanical means for 10minutes.Immediately pass the liquid through a dry filter paper,discarding the first mLof the filtrate.Transfer 1.0mLof the filtrate to a 25-mLvolumetric flask,dilute with 0.1Nhydrochloric acid to volume,and mix.Determine the absorbances of the solution in a 1-cm cell at 236nm and 282nm and at the wavelength of maximum absorbance at about 259nm,using 1.0mLof sodium chloride solution (0.9in 100)diluted to 25mLwith 0.1Nhydrochloric acid as the blank.Calculate the percentage of nicotine released by the formula:
,and shake by mechanical means for 10minutes.Immediately pass the liquid through a dry filter paper,discarding the first mLof the filtrate.Transfer 1.0mLof the filtrate to a 25-mLvolumetric flask,dilute with 0.1Nhydrochloric acid to volume,and mix.Determine the absorbances of the solution in a 1-cm cell at 236nm and 282nm and at the wavelength of maximum absorbance at about 259nm,using 1.0mLof sodium chloride solution (0.9in 100)diluted to 25mLwith 0.1Nhydrochloric acid as the blank.Calculate the percentage of nicotine released by the formula:
(77400/CW)(A259-0.5A236-0.5A282),
in which 77400is a specific absorbance and dilution factor;Cis the percentage of nicotine in the Nicotine Polacrilex on the basis of the amount determined in the Assay;Wis the weight,in mg,of the Nicotine Polacrilex taken;and A259,A236,and A282are the absorbances of the solution under test,corrected for the blank absorbances,at the wavelengths indicated by the subscripts:not less than 70%is released in 10minutes.
Water,Method Iá921ñ—
Transfer about 1.0g of Nicotine Polacrilex,accurately weighed,to a 50-mLglass-stoppered test tube,add 20.0mLof methanol,shake for 30minutes,and allow to stand for about 30minutes.Use a 10-mLportion of the methanol layer for the titration:not more than 5.0%is found.
Chromatographic purity—
Mobile phase andChromatographic system—
Proceed as directed in the Assay.
Standard solution—
Transfer 1.0mLof the Standard preparation,prepared as directed in the Assay,to a 100-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Test solution—
Use the Assay preparation.
Procedure—
[NOTE—Use peak areas where peak responses are indicated.]Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto the chromatograph,and allow the chromatograms to run for not less than two times the retention time of the major peak.Record the chromatograms,and measure the peak responses.Calculate the percentage of each impurity in the portion of Nicotine Polacrilex taken by the formula:
(162.23/462.41)(100/3)(100C/W)(ri/rS),
in which 162.23and 462.41are the molecular weights of nicotine and anhydrous nicotine bitartrate,respectively,Cis the concentration,in mg per mL,of USP Nicotine Bitartrate Dihydrate RSon the anhydrous basis in the Standard solution,Wis the weight,in mg,of nicotine in the Nicotine Polacrilex taken,and riand rSare peak responses for the individual impurity and nicotine bitartrate dihydrate obtained from the Test solutionand the Standard solution,respectively:not more than 0.3%of any individual impurity is found,and the sum of all impurities is not more than 1.0%.
Assay—
0.25MSodium dodecylsulfate—
Dissolve 18.02g of sodium dodecyl sulfate in 25mLof glacial acetic acid and sufficient water to make 250mL,and mix.
Mobile phase—
Mix 640mLof water,50mLof 1Msodium acetate,and 40mLof 0.25M Sodium dodecyl sulfate,and filter.Add 270mLof acetonitrile,mix,and degas.Adjust the amount of water if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity of USP Nicotine Bitartrate Dihydrate RSin 1Mammonium hydroxide to obtain a solution having a known concentration of about 6mg per mL.Transfer 3.0mLof this solution to a 10-mLvolumetric flask,add 3mLof 1Macetic acid,dilute with water to volume,and mix.
Assay preparation—
Transfer an accurately weighed quantity of Nicotine Polacrilex,equivalent to about 20mg of nicotine,to a glass-stoppered test tube.Add 10.0mLof 1Mammonium hydroxide,shake for 10minutes,and centrifuge.Transfer 3.0mLof the clear solution to a 10-mLvolumetric flask,add 3mLof 1Macetic acid,and dilute with water to volume.[NOTE—Retain the resin residue from centrifugation for use in Identification test B.]
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm ×30-cm column containing packing L1.The flow rate is about 2.0mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the tailing factor is not more than 2.0;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the areas of the major peaks.Calculate the percentage of nicotine (C10H14N2)in the portion of Nicotine Polacrilex taken by the formula:
100(162.23/462.41)(100C/3W)(rU/rS),
in which 162.23and 462.41are the molecular weights of nicotine and anhydrous nicotine bitartrate,respectively;Cis the concentration,in mg per mL,of USP Nicotine Bitartrate Dihydrate RSon the anhydrous basis in the Standard preparation;Wis the weight,in mg,of the portion of Nicotine Polacrilex taken;and rUand rSare the peak areas obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Ravi Ravichandran,Ph.D.,Senior Scientist
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 1373
Pharmacopeial Forum:Volume No.28(4)Page 1168
Phone Number:1-301-816-8330