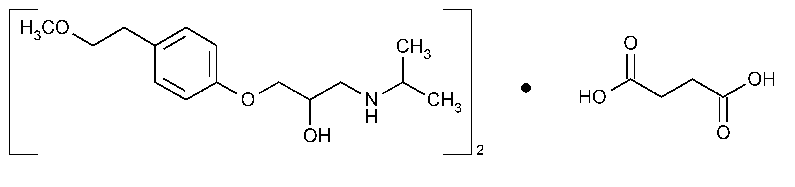
Metoprolol Succinate
»Metoprolol Succinate contains not less than 98.0percent and not more than 102.0percent of (C15H25NO3)2·C4H6O4,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers at controlled room temperature.
Clarity and color of solution—
Asolution of Metoprolol Succinate having a concentration of 20mg per mLis not less clear than an equal volume of water in a test tube of similar size.The absorbance of the solution determined at 440nm in a 5-cm cell,using water as the blank,is not more than 0.1.
Identification,
Infrared Absorption á197Kñ.
pHá791ñ:
between 7.0and 7.6,in a solution containing 65mg per mL.
Loss on drying á731ñ—
Dry it in vacuum at 100 to 105
to 105 for 4hours:it loses not more than 0.2%of its weight.
for 4hours:it loses not more than 0.2%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method Iá231ñ:
0.001%.
Related compounds—
TEST1—
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture.
Test solution—
Dissolve an accurately weighed quantity of Metoprolol Succinate in methanol to obtain a solution containing 50mg per mL.
Standard solution—
Dilute the Test solutionquantitatively,and stepwise if necessary,with methanol to obtain a solution having a concentration of 0.1mg per mL.
Application volume:
10µL.
Developing solvent system:
a mixture of ethyl acetate and methanol (80:20).
Procedure—
Proceed as directed for Thin-Layer Chromatographyunder Chromatography á621ñ.Place two 50-mLbeakers,each containing 30mLof ammonium hydroxide,on the bottom of a chromatographic chamber that is lined with filter paper and contains the Developing solvent system,and allow to equilibrate for l hour.Position the plate in the chromatographic chamber,and develop the chromatogram until the solvent front has moved about two-thirds of the length of the plate.Remove the plate from the chamber,mark the solvent front,and dry the plate for 3hours in a current of warm air.Place the plate in a chamber containing iodine vapor,and allow to react for at least 15hours.Compare the intensities of the brown spots appearing on the chromatogram:any secondary spot obtained from the Test solutionis not more intense than the corresponding spot obtained from the Standard solution.Not more than 0.2%is found.
TEST2—
Sodium dodecyl sulfate solution,Mobile phase,andResolution solution—
Prepare as directed in the Assay.
Standard solution—
Dissolve an accurately weighed quantity of USP Metoprolol Succinate RSin Mobile phase,and dilute quantitatively,and stepwise if necessary,with Mobile phaseto obtain a solution having a known concentration of about 1.0µg per mL.
Test solution—
Transfer about 50mg of Metoprolol Succinate,accurately weighed,to a 50-mLvolumetric flask,dissolve in and dilute with Mobile phaseto volume,and mix.
Chromatographic system (seeChromatography á621ñ)—
Prepare as directed in the Assay.Chromatograph the Resolution solution,and record the peak responses as directed for Procedure:the resolution,R,between metoprolol related compound Aand metoprolol related compound Bis not less than 1.5;and the resolution,R,between metoprolol related compound Band metoprolol related compound Cis not less than 2.5.[NOTE—The relative retention times are about 0.6for metoprolol related compound C,0.7for metoprolol related compound B,0.8for metoprolol related compound A,1.0for metoprolol,and 5.0and 5.2for the two diastereomers of metoprolol related compound D.]Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 5.0%.
Procedure—
Inject equal volumes (about 10µL)of the Standard solutionand the Test solution into the chromatograph,record the chromatograms,and measure the peak responses.Calculate the percentage of each impurity in the portion of metoprolol succinate taken by the formula:
(CS/CT)(ri/rS),
in which CSis the concentration,in mg per mL,of USP Metoprolol Succinate RSin the Standard solution;CTis the concentration of metoprolol succinate in the Test solution;riis the individual peak response of related impurities;and rSis the peak response obtained from the Standard solution:not more than 0.1%of any single impurity is found,and not more than 0.5%of total impurities is found.[NOTE—The sum of the peak responses for the two diastereomers of metoprolol related compound Dis used in the above calculation to report the amount of metoprolol related compound D.]
Assay—
Sodium dodecyl sulfate solution—
Add 1.3g of sodium dodecyl sulfate to 1liter of aqueous phosphoric acid,0.1%(w/v).
Mobile phase—
Prepare a filtered and degassed mixture of Sodium dodecyl sulfate solutionand acetonitrile (60:40).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Resolution solution—
Prepare a solution in Mobile phasecontaining about 5µg each of USP Metoprolol Succinate RS,USP Metoprolol Related Compound A RS,USP Metoprolol Related Compound B RS,USP Metoprolol Related Compound C RS,and USP Metoprolol Related Compound D RSper mL.
Standard preparation—
Dissolve an accurately weighed quantity of USP Metoprolol Succinate RSin Mobile phase,and dilute quantitatively,and stepwise if necessary,with Mobile phaseto obtain a solution having a known concentration of about 0.08mg per mL.
Test preparation—
Transfer about 80mg of Metoprolol Succinate,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with Mobile phaseto volume,and mix.Transfer 5.0mLof this solution to a 50-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Chromatographic system (seeChromatography á621ñ)—
The liquid chromatograph is equipped with a 223-nm detector and a 4-mm ×12.5-cm column that contains 4-µm packing L7.The column temperature is maintained at 30 .The flow rate is about 0.9mLper minute.Chromatograph the Resolution solution,and record the peak responses as directed for Procedure:the resolution,R,between metoprolol related compound Aand metoprolol related compound Bis not less than 1.5;and the resolution,R,between metoprolol related compound Band metoprolol related compound Cis not less than 2.5.[NOTE—The relative retention times are about 0.6for metoprolol related compound C,0.7for metoprolol related compound B,0.8for metoprolol related compound A,1.0for metoprolol,and 5.0and 5.2for the two diastereomers of metoprolol related compound D.]Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 2.0%.
.The flow rate is about 0.9mLper minute.Chromatograph the Resolution solution,and record the peak responses as directed for Procedure:the resolution,R,between metoprolol related compound Aand metoprolol related compound Bis not less than 1.5;and the resolution,R,between metoprolol related compound Band metoprolol related compound Cis not less than 2.5.[NOTE—The relative retention times are about 0.6for metoprolol related compound C,0.7for metoprolol related compound B,0.8for metoprolol related compound A,1.0for metoprolol,and 5.0and 5.2for the two diastereomers of metoprolol related compound D.]Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Inject equal volumes (about 10µL)of the Standard preparationand the Test preparationinto the chromatograph,record the chromatograms for at least 1.5times the retention of the metoprolol peak,and measure the peak responses.Calculate the quantity,in mg,of (C15H25NO3)2·C4H6O4in the portion of Metoprolol Succinate taken by the formula:
1000C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Metoprolol Succinate RSin the Standard preparation;and rUand rSare the peak responses obtained from the Test preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 1279
Pharmacopeial Forum:Volume No.30(4)Page 1263
Phone Number:1-301-816-8305