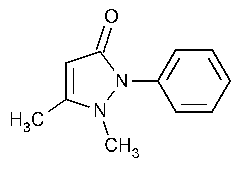
Antipyrine
1,2-Dihydro-1,5-dimethyl-2-phenyl-3H-pyrazol-3-one.
2,3-Dimethyl-1-phenyl-3-pyrazolin-5-one [60-80-0].
»Antipyrine contains not less than 99.0percent and not more than 100.5percent of C11H12N2O,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Completeness and color of solution—
It is completely soluble in its own weight of cold water,the solution being colorless or not more than slightly yellow when viewed transversely in a tube having a diameter of about 20mm.
Identification—
A:
Infrared Absorption á197Kñ.
B:
Ultraviolet Absorption á197Uñ—
Solution:
20µg per mL.
Medium:
methanol.
Absorptivities at 266nm,calculated on the dried basis,do not differ by more than 3.0%.
C:
Add tannic acid TSto a solution of it:a white precipitate is formed.
Loss on drying á731ñ—
Dry it at 60 for 2hours:it loses not more than 1.0%of its weight.
for 2hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.15%.
Heavy metals á231ñ—
Dissolve 1g in 2mLof 1Nacetic acid,and add water to make 25mL:the limit is 0.002%.
Ordinary impurities á466ñ—
Test solution:
chloroform.
Standard solution:
chloroform.
Eluant:
a mixture of chloroform,acetone,butyl alcohol,and formic acid (60:15:15:15).
Visualization:
1.
Assay—
Transfer about 150mg of Antipyrine,accurately weighed,to a 250-mLiodine flask,and dissolve in 25mLof water.Add 2g of sodium acetate,1mLof diluted acetic acid,and 20.0mLof 0.1Niodine VS,mix,and allow to stand in a cool,dark place for 20minutes.Add 25mLof alcohol to dissolve the precipitate,and titrate the excess iodine with 0.1Nsodium thiosulfate VS,using starch TSas the indicator.Each mLof 0.1Niodine is equivalent to 9.412mg of C11H12N2O.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA2)Pharmaceutical Analysis 2
USP28–NF23Page 169
Phone Number:1-301-816-8139