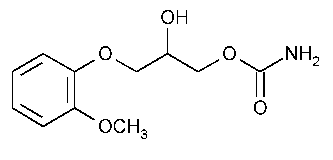
Methocarbamol
1,2-Propanediol,3-(2-methoxyphenoxy)-,1-carbamate,(±)-.
(±)-3-(o-Methoxyphenoxy)-1,2-propanediol 1-carbamate [532-03-6].
»Methocarbamol contains not less than 98.5percent and not more than 101.5percent of C11H15NO5,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
Infrared Absorption á197Kñ.
Solution:
40µg per mL.
Medium:
alcohol.
Loss on drying á731ñ—
Dry it at 60 for 2hours:it loses not more than 0.5%of its weight.
for 2hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method Iá231ñ—
Dissolve 1.0g in a mixture of 7mLof methanol and 3mLof 1Nacetic acid,and dilute with water to 25mL.The limit is 0.002%.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Chromatographic purity—
pH4.5Buffer solution—
Dissolve 6.8g of monobasic potassium phosphate in 1000mLof water.Adjust with 18Nphosphoric acid or 10Npotassium hydroxide to a pHof 4.5±.05.
Mobile phase—
Prepare a suitably filtered and degassed solution of pH4.5Buffer solutionand methanol (about 75:25)(see System suitability).
Guaifenesin solution—
Transfer 20.0mg of USP Guaifenesin RSto a 50-mLvolumetric flask.Dissolve in and dilute with methanol to volume,and mix.
Standard solution—
Transfer 20.0mg of USP Methocarbamol RS,accurately weighed,to a 10-mLvolumetric flask.Add 1.0mLof Guaifenesin solutionand 2.0mLof methanol to dissolve the methocarbamol.Dilute with pH4.5Buffer solutionto volume,and mix.Use this solution within 24hours.
Test solution—
Transfer about 100mg of Methocarbamol,accurately weighed,to a 50-mLvolumetric flask,add 13mLof methanol to dissolve,dilute with pH4.5Buffer solutionto volume,and mix.Use this solution within 24hours.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 274-nm detector and a 4-mm ×25-cm column that contains packing L1.Adjust the operating conditions so that System suitabilityrequirements are met.
System suitability—
Chromatograph three replicate 20-µLportions of the Standard solutionas directed for the Test solutionunder Procedure.The analytical system is suitable for use if the peak area percentage of guaifenesin is 2.4±1.0,the relative standard deviation for the peak area percentage is not greater than 4.0%,and the resolution,R,between guaifenesin and methocarbamol is not less than 2.0.
Procedure—
By means of a suitable microsyringe or sampling valve,inject about 20µLof the Test solutioninto the chromatograph.Determine the peak areas of the methocarbamol peak and all extraneous peaks having a retention time greater than 0.5of the retention time of methocarbamol.The relative retention times are about 0.8for guaifenesin and 1.0for methocarbamol.Calculate the percentage of related impurities taken by the formula:
100(2.4/G)(PE/PT),
in which Gis the area percentage of the guaifenesin peak in the chromatogram of the Standard solutiondetermined under System suitability;PEis the peak area of all extraneous peaks;and PTis the total of the peak areas of all extraneous peaks and the methocarbamol.The limit is 2.0%.
Assay—
Transfer about 100mg of Methocarbamol,accurately weighed,to a 100-mLvolumetric flask,add methanol to volume,and mix.Transfer 4.0mLof this solution to a second 100-mLvolumetric flask,dilute with methanol to volume,and mix.Concomitantly determine the absorbances of this solution and a Standard solution of USP Methocarbamol RSin methanol having a known concentration of about 40µg per mLat the wavelength of maximum absorbance at about 274nm in 1-cm cells,using methanol as the blank.Calculate the quantity,in mg,of C11H15NO5in the portion of Methocarbamol taken by the formula:
2.5C(AU/AS),
in which Cis the concentration,in µg per mL,of USP Methocarbamol RSin the Standard solution;and AUand ASare the absorbances of the Assay solutionand the Standard solution,respectively.
Auxiliary Information—
Staff Liaison:Ravi Ravichandran,Ph.D.,Senior Scientist
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 1245
Pharmacopeial Forum:Volume No.29(6)Page 1930
Phone Number:1-301-816-8330