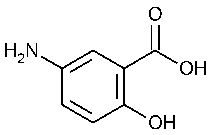
Mesalamine
»Mesalamine contains not less than 98.5percent and not more than 101.5percent of C7H7NO3,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Clarity of solution—
Afreshly prepared solution of 0.25g of it in 10mLof 1Nhydrochloric acid is clear.
Identification—
A:
Infrared Absorption á197Kñ.
B:
Ultraviolet Absorption á197Uñ—
Solution:
12µg per mL.
Medium:
0.1Nhydrochloric acid.
Absorptivities at 230nm do not differ by more than 3.0%.
C:
Dissolve about 100mg of it in 5mLof 0.1Nhydrochloric acid,and add 1drop of ferric chloride TS:a purplish violet color is produced.
pHá791ñ:
between 3.5and 4.5,in a suspension (1in 40).
Loss on drying á731ñ—
Dry it in vacuum at 105 for 3hours:it loses not more than 0.5%of its weight.
for 3hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.2%.
Chloride á221ñ—
Disperse 500mg in 40mLof water,sonicate for 5minutes,and filter the dispersion.To the filtrate add 1mLof nitric acid:the solution shows no more chloride than corresponds to 0.7mLof 0.020Nhydrochloric acid (0.1%).
Heavy metals,Method IIá231ñ:
0.002%.
Hydrogen sulfide and sulfur dioxide—
Dissolve about 500mg in 5mLof 1Nsodium hydroxide,add 6mLof 3Nhydrochloric acid,and stir vigorously.Apiece of moistened lead acetate test paper held over the mixture does not become discolored.
Sulfate—
Proceed as directed in the test for Sulfate á221ñ:a 500-mg portion shows no more sulfate than corresponds to 1.0mLof 0.02Nsulfuric acid (0.2%).
Related compounds—
TEST1
(for 3-aminosalicylic acid and other related impurities)—[NOTE—Use Test 1to measure 3-aminosalicylic acid and other related impurities not measured in Test 2.]
Mobile phase—
Dissolve 1.36g of monobasic potassium phosphate and 2.2g of sodium 1-octanesulfonate in 890mLof water,and adjust with phosphoric acid to a pHof 2.2.Pass through a filter having 0.5-µm or finer porosity.To the filtrate add 80mLof methanol and 30mLof acetonitrile.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard solution—
Dissolve accurately weighed quantities of USP Mesalamine RSand 3-aminosalicylic acid quantitatively in Mobile phaseto obtain a solution having known concentrations of about 1µg of each per mL.
Test solution—
Transfer about 50mg of Mesalamine,accurately weighed,to a 100-mLvolumetric flask,add about 75mLof Mobile phase,and sonicate briefly to dissolve.Dilute with Mobile phaseto volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm ×15-cm column that contains 5-µm packing L7.The flow rate is about 1.2mLper minute.Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative retention times are about 1.0for mesalamine and 1.3for 3-aminosalicylic acid;and the resolution,R,between the mesalamine peak and the 3-aminosalicylic acid peak is not less than 2.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms for a period of time that is three times the retention time of mesalamine,and measure the peak area responses.Calculate the percentage of 3-aminosalicylic acid by the formula:
0.2C3(r3/rS3),
in which C3is the concentration,in µg per mL,of 3-aminosalicylic acid in the Standard solution;r3is the response of the 3-aminosalicylic acid peak in the chromatogram obtained from the Test solution;and rS3is the response of the 3-aminosalicylic acid peak in the chromatogram obtained from the Standard solution.Calculate the percentage of each other impurity by the formula:
0.2Cm(ri/rSm),
in which Cmis the concentration,in µg per mL,of USP Mesalamine RSin the Standard solution;riis the response of the individual impurity peak in the chromatogram obtained from the Test solution;and rSmis the response of the mesalamine peak in the chromatogram obtained from the Standard solution:not more than 0.2%of 3-aminosalicylic acid is found;not more than 0.2%of any other impurity,expressed in terms of mesalamine equivalent,is found;and the total of all impurities found is not more than 1.0%.
TEST2(for aniline,2-aminophenol,and 4-aminophenol)—
Standard solution—
Prepare a solution of aniline,2-aminophenol,and 4-aminophenol in methanol having concentrations of 0.05,2,and 2mg per mL,respectively,and dilute quantitatively and stepwise,if necessary,with methylene chloride to obtain a solution having known concentrations of 0.5,20,and 20µg per mL,respectively.
Test solution—
Mix 1.0g of Mesalamine with 10.0mLof methylene chloride.Allow to settle,and use the clear methylene chloride solution as the Test solution.
Chromatographic system
(see Chromatography á621ñ)—The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm ×10-m fused-silica capillary column coated with a 2.65-µm film of stationary phase G27.The carrier gas is helium flowing at a rate of 1.5mLper minute.The injector port and the detector are maintained at about 280 and 300
and 300 ,respectively.The column temperature is programmed according to the following steps:the starting column temperature is 70
,respectively.The column temperature is programmed according to the following steps:the starting column temperature is 70 ;after injection it is held at 70
;after injection it is held at 70 for 2minutes,then increased to 150
for 2minutes,then increased to 150 at a rate of 30
at a rate of 30 per minute,then held for 1minute.Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.5for aniline,0.9for 2-aminophenol,and 1.0for 4-aminophenol;and the peaks are baseline separated.
per minute,then held for 1minute.Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.5for aniline,0.9for 2-aminophenol,and 1.0for 4-aminophenol;and the peaks are baseline separated.
Procedure—
Separately inject equal volumes (about 2µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the peak area responses.Identify by retention time any peaks present in the chromatogram of the Test solutionthat correspond to those in the chromatogram obtained from the Standard solution.Calculate the quantities,in µg per g,of aniline,2-aminophenol,and 4-aminophenol in the portion of Mesalamine taken by the formula:
10C(ra/rSa),
in which Cis the concentration,in µg per mL,of the relevant analyte in the Standard solution;rais the response of the relevant analyte in the chromatogram obtained from the Test solution;and rSais the response of the relevant analyte in the chromatogram obtained from the Standard solution:not more than 5µg of aniline,200µg of 2-aminophenol,and 200µg of 4-aminophenol per g are found.
Assay—
Buffer solution—
Transfer 7.1g of anhydrous dibasic sodium phosphate and 6.9g of monobasic sodium phosphate to a 1000-mLvolumetric flask,add 500mLof water,and swirl to dissolve.Add 7.5mLof a solution of tetrabutylammonium hydroxide in methanol (1in 4),dilute with water to volume,and mix.
Mobile phase—
Prepare a suitable degassed mixture of Buffer solutionand methanol (85:15).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Resolution solution—
Prepare a solution in Mobile phasecontaining about 0.25mg of 4-aminosalicylic acid and 0.4mg of USP Mesalamine RSper mL.
Standard preparation—
Dissolve an accurately weighed quantity of USP Mesalamine RSquantitatively in Mobile phaseto obtain a solution having a known concentration of about 1mg per mL.Transfer 10.0mLof this solution to a 25-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.This solution contains about 0.4mg of USP Mesalamine RSper mL.
Assay preparation—
Transfer about 50mg of Mesalamine,accurately weighed,to a 50-mLvolumetric flask,dissolve in and dilute with Mobile phaseto volume,and mix.Transfer 10.0mLof this solution to a 25-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 254-nm detector and a 4-mm ×30-cm column that contains packing L1.The flow rate is about 2mLper minute.Chromatograph the Resolution solution,and record the peak responses as directed for Procedure:the resolution,R,between the 4-aminosalicylic acid and mesalamine peaks is not less than 2.0.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the tailing factor is not more than 2.5;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 15µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of C7H7NO3in the portion of Mesalamine taken by the formula:
125C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Mesalamine RSin the Standard preparation;and rUand rSare the responses of the mesalamine peaks obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Daniel K.Bempong,Ph.D.,Scientist
Expert Committee:(PA2)Pharmaceutical Analysis 2
USP28–NF23Page 1220
Phone Number:1-301-816-8143