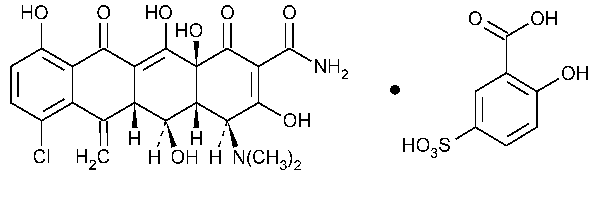
Meclocycline Sulfosalicylate
2-Naphthacenecarboxamide,7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-,[4S-(4a,4aa,5a,5aa,12aa)]-,mono(2-hydroxy-5-sulfobenzoate)(salt).
(4S,4aR,5S,5aR,12aS)-7-Chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-2-naphthacene carboxamide mono(5-sulfosalicylate)(salt) [73816-42-9].
»Meclocycline Sulfosalicylate has a potency equivalent to not less than 620µg of meclocycline (C22H21ClN2O8)per mg.
Packaging and storage—
Preserve in tight containers,protected from light.
Identification,Infrared Absorption á197Kñ.
Crystallinity á695ñ:
meets the requirements.
pHá791ñ:
between 2.5and 3.5,in a solution containing 10mg per mL.
Water,Method Iá921ñ:
not more than 4.0%.
Assay—
0.001M Ammonium edetate—
Transfer 293mg of edetic acid,accurately weighed,to a 1000-mLvolumetric flask,add 1mLof methanol and 7mLof ammonium hydroxide,and shake to dissolve the edetic acid.Add 900mLof water,adjust with glacial acetic acid to a pHof 6.6,dilute with water to volume,and mix.
Mobile phase—
Prepare a solution of 0.001M Ammonium edetateand tetrahydrofuran (85:15).Filter and degas the solution before use.
Standard preparation—
Transfer 36mg of USP Meclocycline Sulfosalicylate RS,accurately weighed,to a 50-mLvolumetric flask,dilute with methanol to volume,and mix.Transfer 3.0mLof this solution to a 25-mLvolumetric flask,dilute with Mobile phaseto volume,and mix to obtain a solution having a known concentration of about 60µg of meclocycline per mL.
Assay preparation—
Using 36mg of Meclocycline Sulfosalicylate,accurately weighed,prepare as directed for Standard preparation.
Procedure—
Introduce equal volumes (about 10µL)of the Assay preparationand the Standard preparationinto a high-pressure liquid chromatograph (see Chromatography á621ñ),operated at room temperature,by means of a suitable microsyringe or sampling valve,adjusting the specimen size and other operating parameters such that the peak obtained from the Standard preparationis about 0.6full-scale.Typically,the apparatus is fitted with a 25-cm ×4-mm column packed with packing L1and is equipped with an UVdetector capable of monitoring absorption at 340nm,and a suitable recorder.In a suitable chromatogram the coefficient of variation for replicate injections of the Standard preparationis not more than 3.0%.Measure the peak responses at equivalent retention times,obtained from the Assay preparationand the Standard preparation,and calculate the quantity,in mg,of C22H21ClN2O8in the portion of Meclocycline Sulfosalicylate taken by the formula:
(1.25/3)(C)(rU/rS),
in which Cis the equivalent,in µg per mL,of meclocycline from the USP Meclocycline Sulfosalicylate RSin the Standard preparation;and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1195
Phone Number:1-301-816-8335